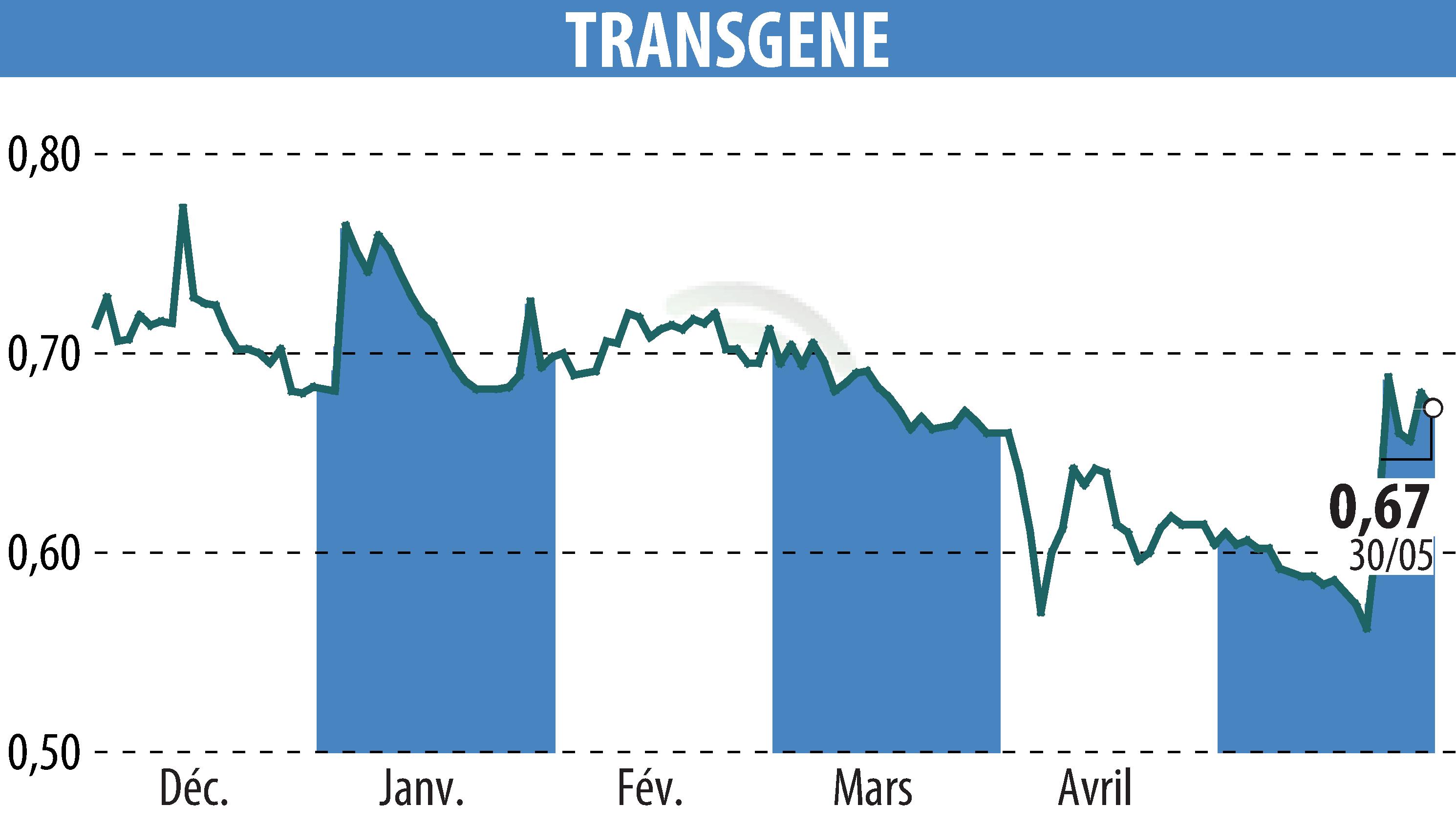
on TRANSGENE (EPA:TNG)
Transgene and NEC: Promising progress with the TG4050 vaccine
Transgene and NEC announce encouraging results for the therapeutic vaccine TG4050, developed for head and neck cancers. Phase I data show that all treated patients are in remission and recurrence-free after two years. TG4050 targets specific neoantigens, inducing a durable CD8+ T cell response.
At the ASCO 2025 conference, results demonstrated promising safety and efficacy. The vaccine, based on NEC's myvac® platform and AI, appears well tolerated with no unexpected adverse effects.
Development of TG4050 continues in Phase II, with the goal of confirming initial results in a larger population. Researchers hope to expand the use of this personalized treatment in adjuvant settings.
R. E.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all TRANSGENE news
