on THERACLION (EPA:ALTHE)
Theraclion obtains crucial certification in China
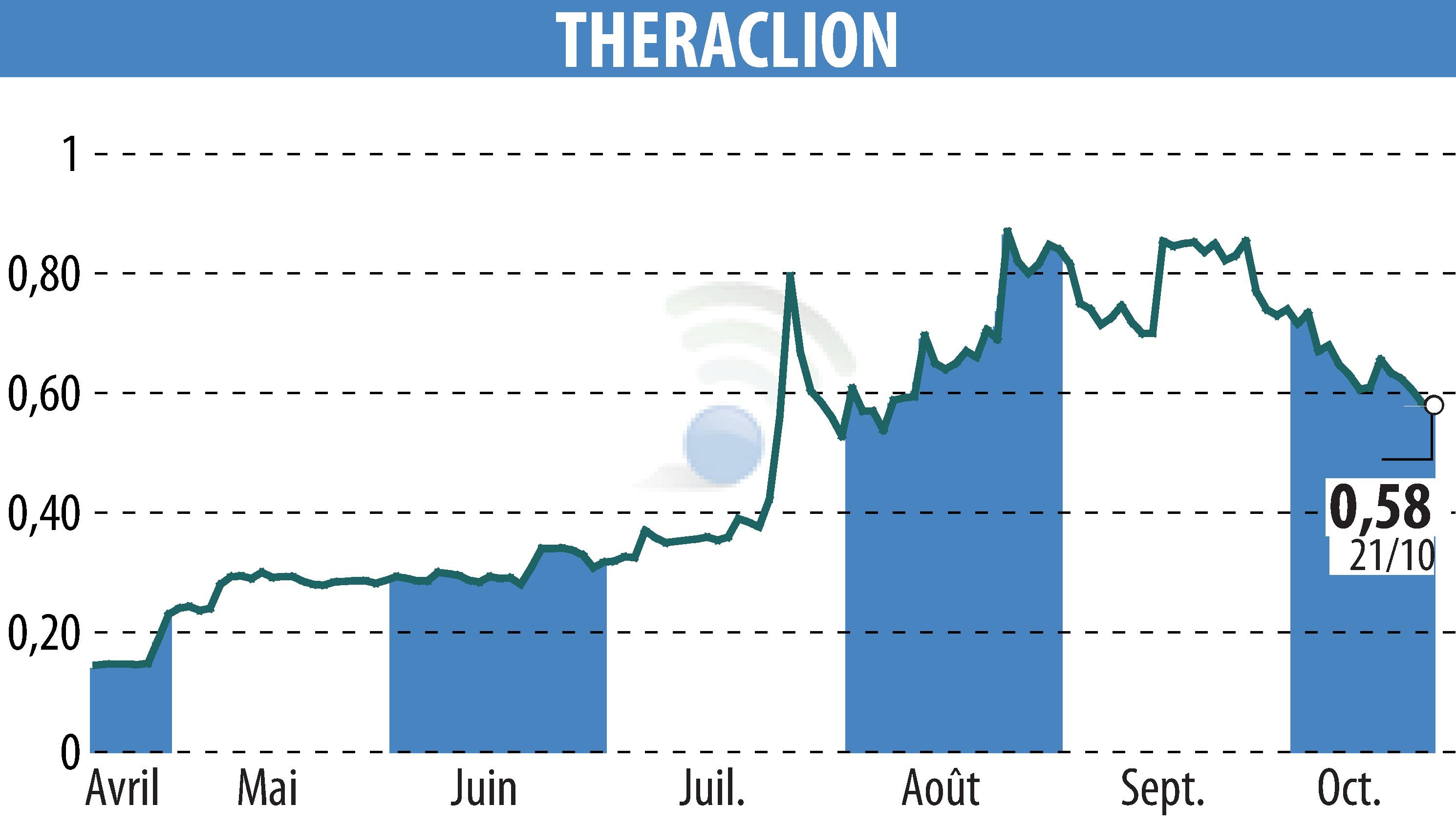
Theraclion, an innovative medical technology company, announces that it has obtained a major Chinese certification for its Sonovein® device. The device has met the requirements of Chinese standard GB 9706.1-2020, relating to the safety of medical electrical equipment. This milestone is crucial for approval by the National Medical Products Administration (NMPA) in China.
This certification validates Sonovein®'s electrical safety and focused ultrasound emissions, confirming its compliance with the NMPA's stringent safety criteria. This is a significant step toward entering the Chinese market, one of the world's largest for advanced medical technologies.
Theraclion continues its expansion strategy with this advancement, aiming to offer a wider audience the benefits of its non-invasive ultrasound. The launch of Sonovein® in China is now closer.
R. E.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all THERACLION news
