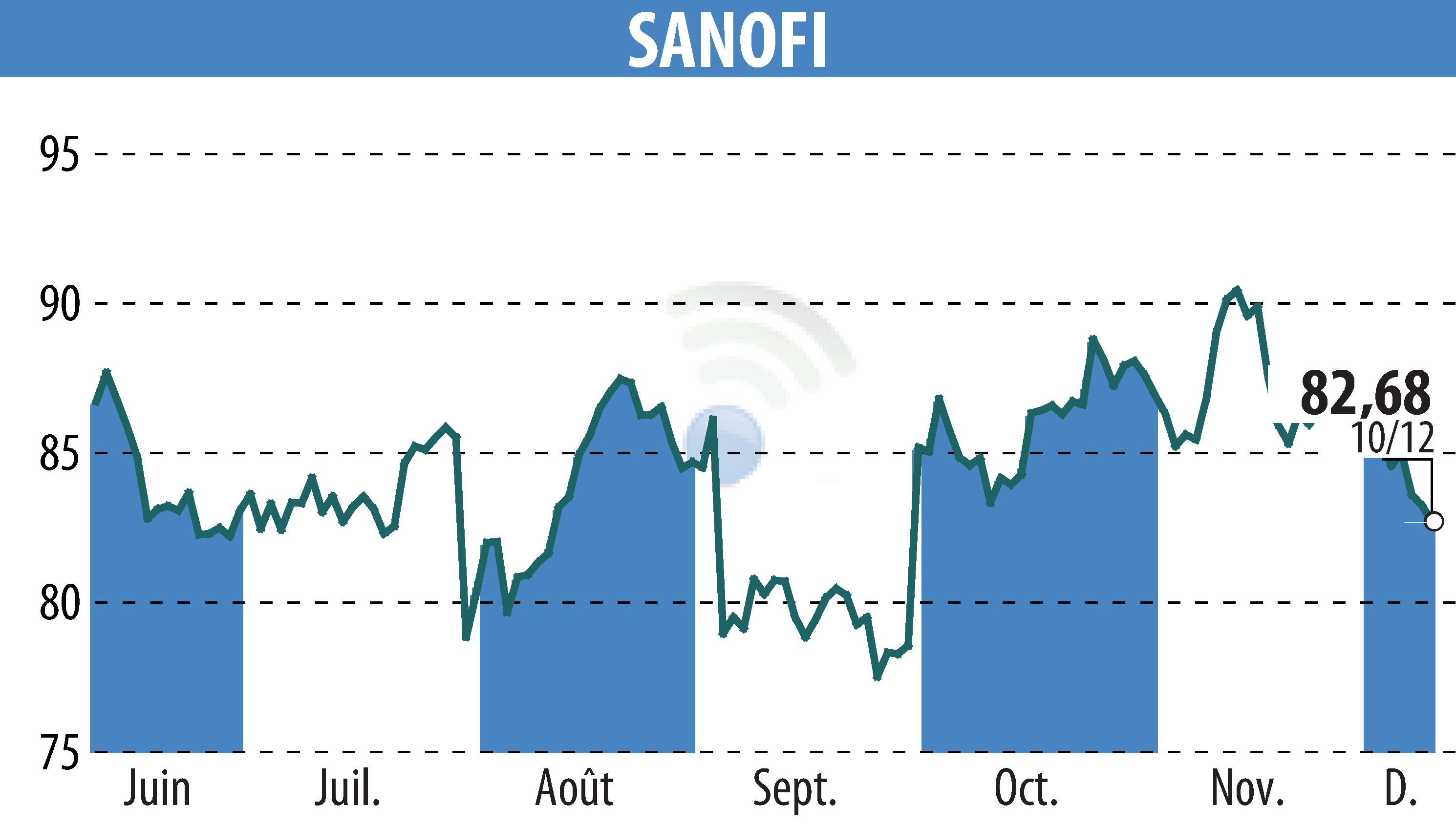
on SANOFI-AVENTIS (EPA:SAN)
Sanofi's Breakthrough in China with Qfitlia and Cablivi Approvals
The National Medical Products Administration (NMPA) of China has approved Sanofi's Qfitlia and Cablivi for rare hematologic diseases. Qfitlia, an antithrombin-lowering therapy, is designed to prevent bleeding in hemophilia patients with just six injections annually. Cablivi targets acquired thrombotic thrombocytopenic purpura (aTTP), a rare blood clotting disorder.
These approvals mark Sanofi's fourth and fifth in China for 2025. Qfitlia's approval follows significant clinical success demonstrated in ATLAS phase 3 trials, showcasing a 71% reduction in bleeding episodes for patients without inhibitors. Meanwhile, Cablivi's approval is significant for China's estimated 2,700 annual aTTP cases, promising improved management of this condition.
R. P.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all SANOFI-AVENTIS news
