on SANOFI-AVENTIS (EPA:SAN)
Sanofi's Rezurock Recommended for EU Approval by CHMP
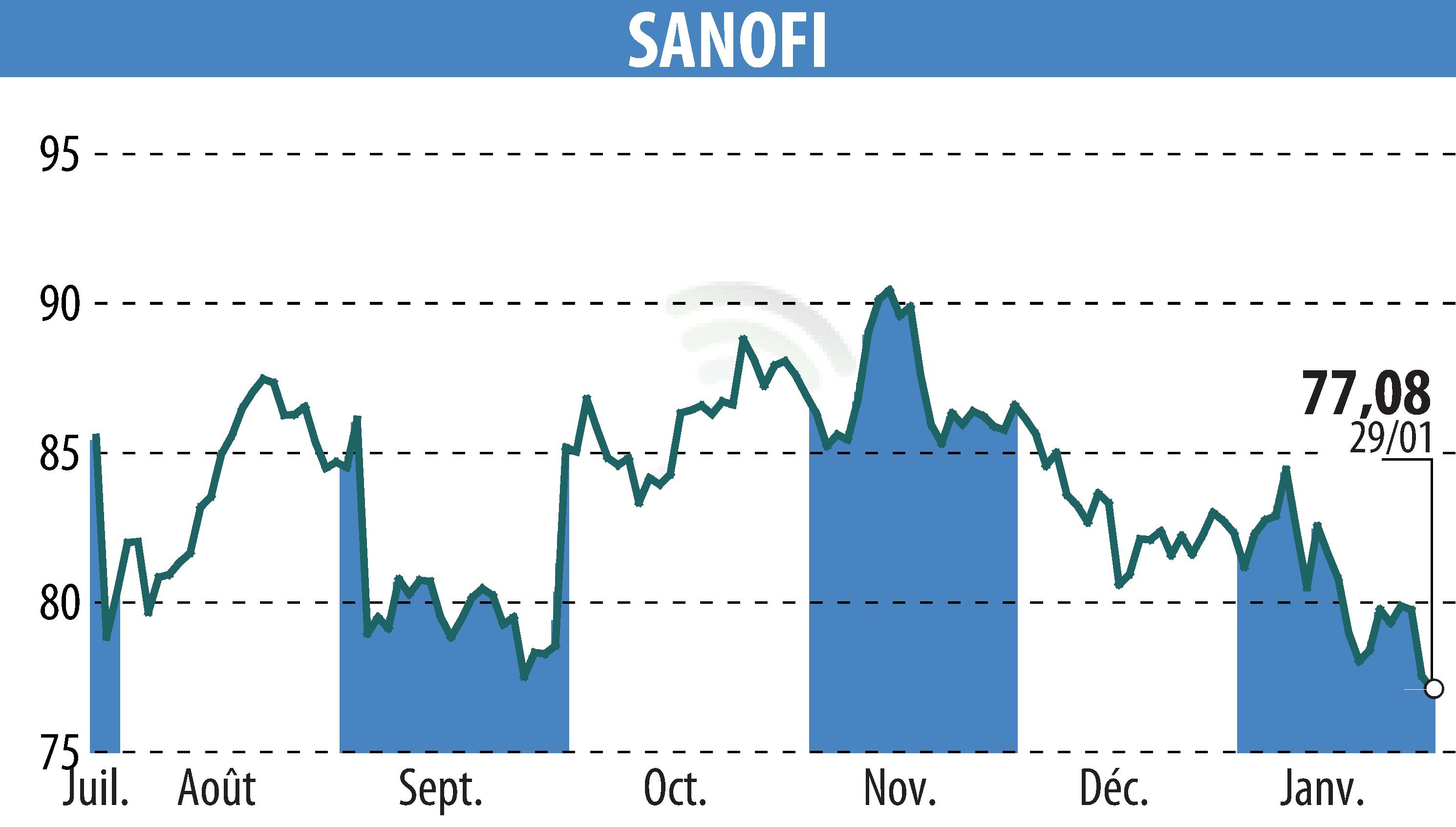
Sanofi's Rezurock, a treatment for chronic graft-versus-host disease (GVHD), has been recommended for conditional EU approval by the Committee for Medicinal Products for Human Use (CHMP). This follows a positive opinion from the European Medicines Agency, supporting its safety and efficacy from multiple studies and real-world evidence. If sanctioned, Rezurock would offer a new option for both adults and children aged 12 and older suffering from late-line chronic GVHD.
This recommendation is significant, following Sanofi's appeal against a previous negative opinion by the CHMP in October 2025. It is backed by data from the phase 2 ROCKstar study, which showed promising results.
Rezurock is already approved in over 20 countries, including the U.S. and Canada, and this positive CHMP opinion could mean its EU launch is imminent once approved by the European Commission.
R. P.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all SANOFI-AVENTIS news
