on SANOFI-AVENTIS (EPA:SAN)
Sanofi Obtains Fast Track Designation for SAR402663 in the United States
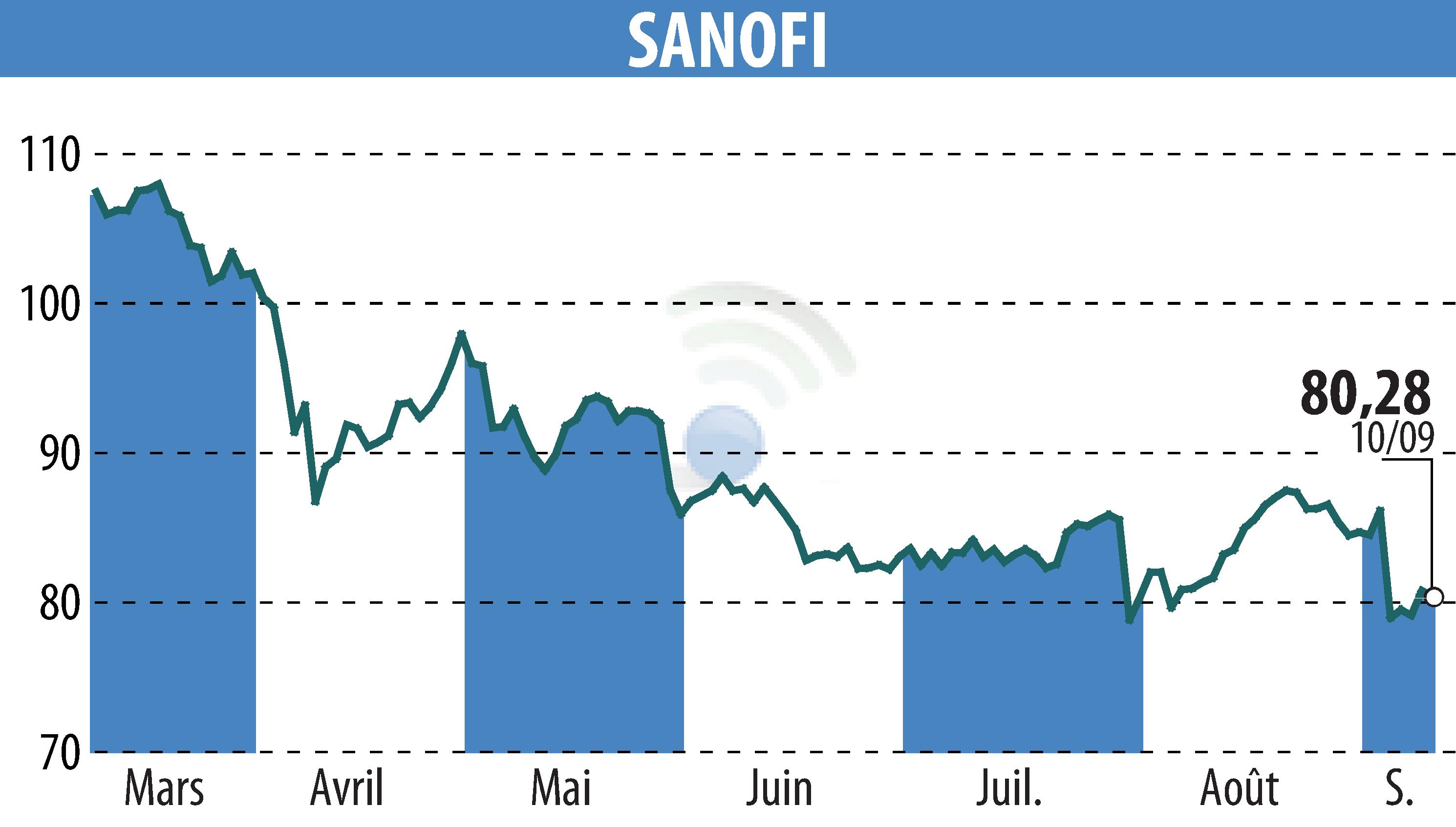
Sanofi announced that the U.S. Food and Drug Administration (FDA) has granted Fast Track designation to its product SAR402663. This innovative gene therapy is intended for the treatment of neovascular age-related macular degeneration (AMD). This serious condition can lead to significant vision loss, affecting more than one million Americans. The designation will facilitate and expedite the development and review of this drug, designed to inhibit the abnormal growth of blood vessels in the eye.
Currently in Phase I/II clinical trials, Sanofi is evaluating the efficacy of SAR402663 in reducing vascular leakage and retinal damage. This initiative is part of the company's efforts to address unmet medical needs and improve patient quality of life. With a single administration, the treatment could replace repeated intravitreal injections, simplifying patient care.
R. H.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all SANOFI-AVENTIS news
