on SANOFI-AVENTIS (EPA:SAN)
Sanofi and Teva: Promising advance in the treatment of inflammatory bowel diseases
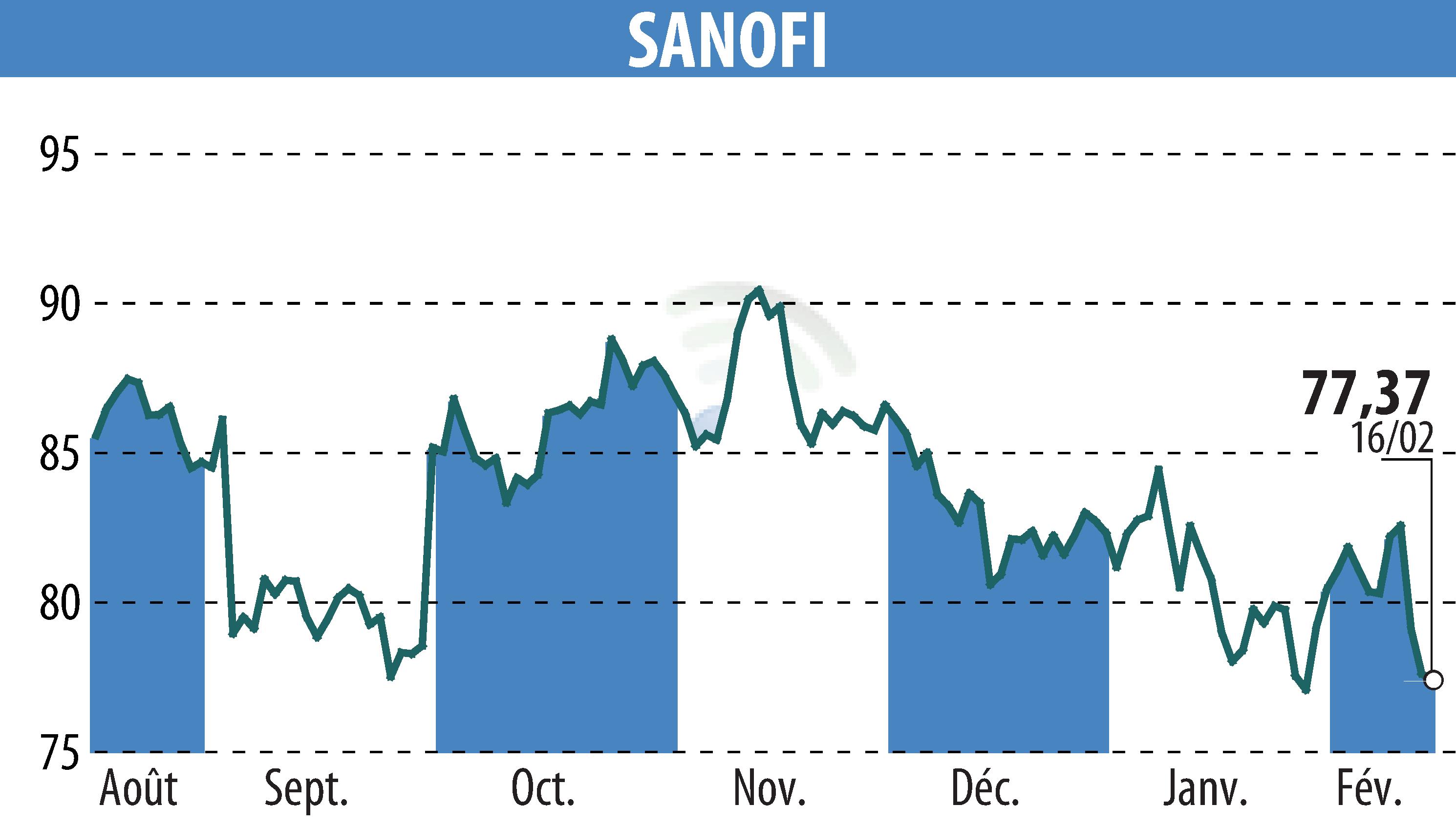
Sanofi and Teva announced positive results from the Phase IIb RELIEVE UCCD LTE study of duvakitug, an investigational monoclonal antibody. This study demonstrated sustained efficacy over 44 weeks in patients with ulcerative colitis (UC) and Crohn's disease (CD) who responded to the initial 14-week induction phase.
Duvakitug, administered every four weeks, achieved significant rates of clinical remission and endoscopic response. It was well tolerated with a safety profile consistent with previous studies. These results could strengthen the potential of duvakitug in future phase III trials.
This treatment is being developed in response to the urgent need for innovative solutions for UC and CD, two common forms of chronic inflammatory bowel disease (IBD).
R. E.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all SANOFI-AVENTIS news
