on SANOFI-AVENTIS (EPA:SAN)
Sanofi and Regeneron's Dupixent Gains Approval in Japan for Young Asthma Patients
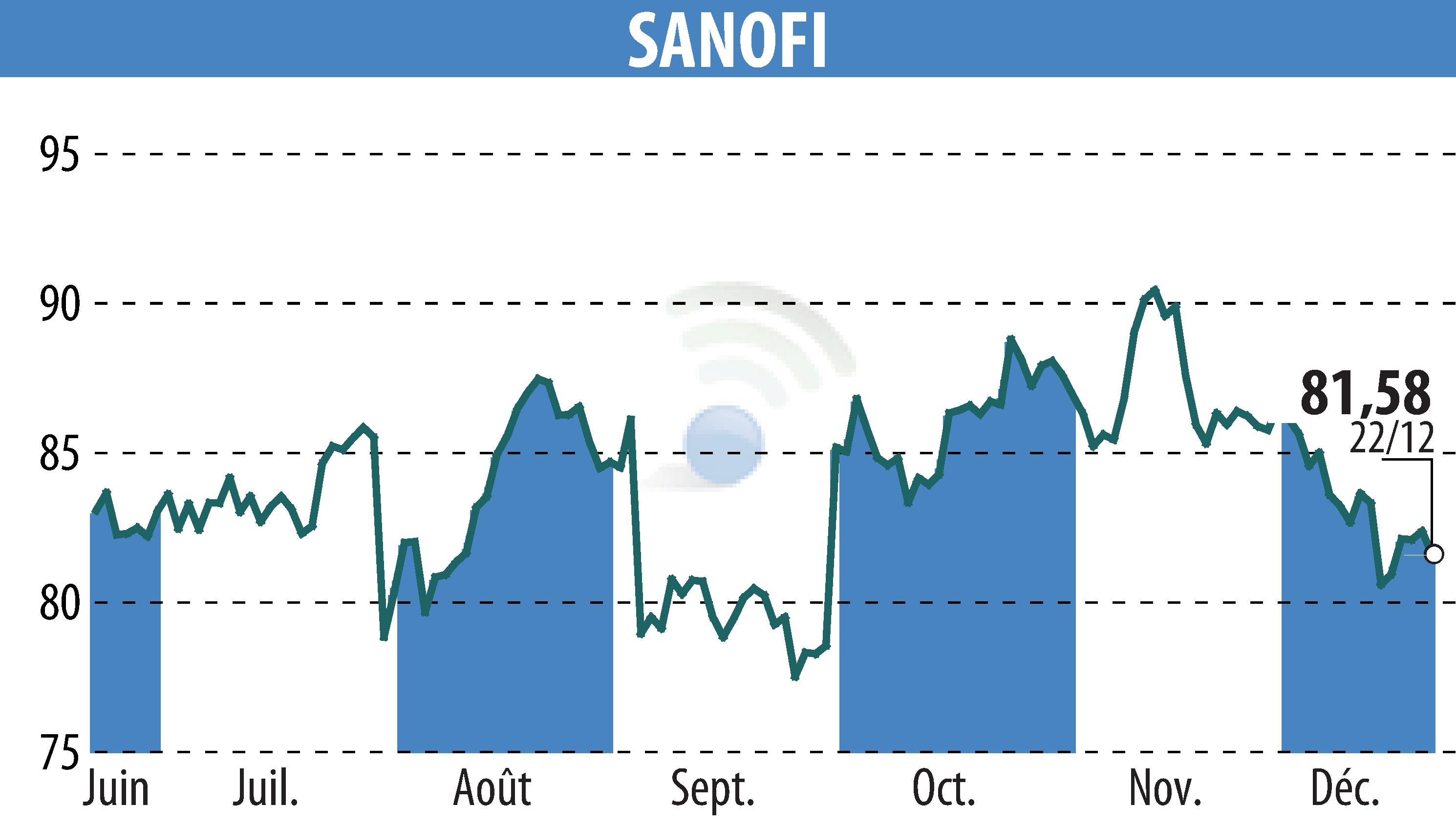
Sanofi and Regeneron have announced that their biologic medication Dupixent has received approval from Japan's Ministry of Health, Labour and Welfare for use in children aged 6 to 11 with severe bronchial asthma. This decision expands the previous authorization for patients aged 12 and above.
The approval is grounded on global phase 3 studies demonstrating Dupixent's efficacy. The trials showed a significant reduction in asthma exacerbations and improved lung function in this young demographic.
Dupixent targets type 2 inflammation by inhibiting interleukin-4 and interleukin-13, pivotal in asthma's pathology. Common side effects include injection site reactions and mild systemic symptoms.
Beyond asthma, Dupixent is approved for multiple conditions in Japan, reinforcing its therapeutic versatility. This development further marks the drug's availability across 50 countries for pediatric asthma treatment.
R. H.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all SANOFI-AVENTIS news
