on Protagonist Therapeutics, Inc. (NASDAQ:PTGX)
Protagonist Therapeutics' Second Quarter 2025 Financial Results
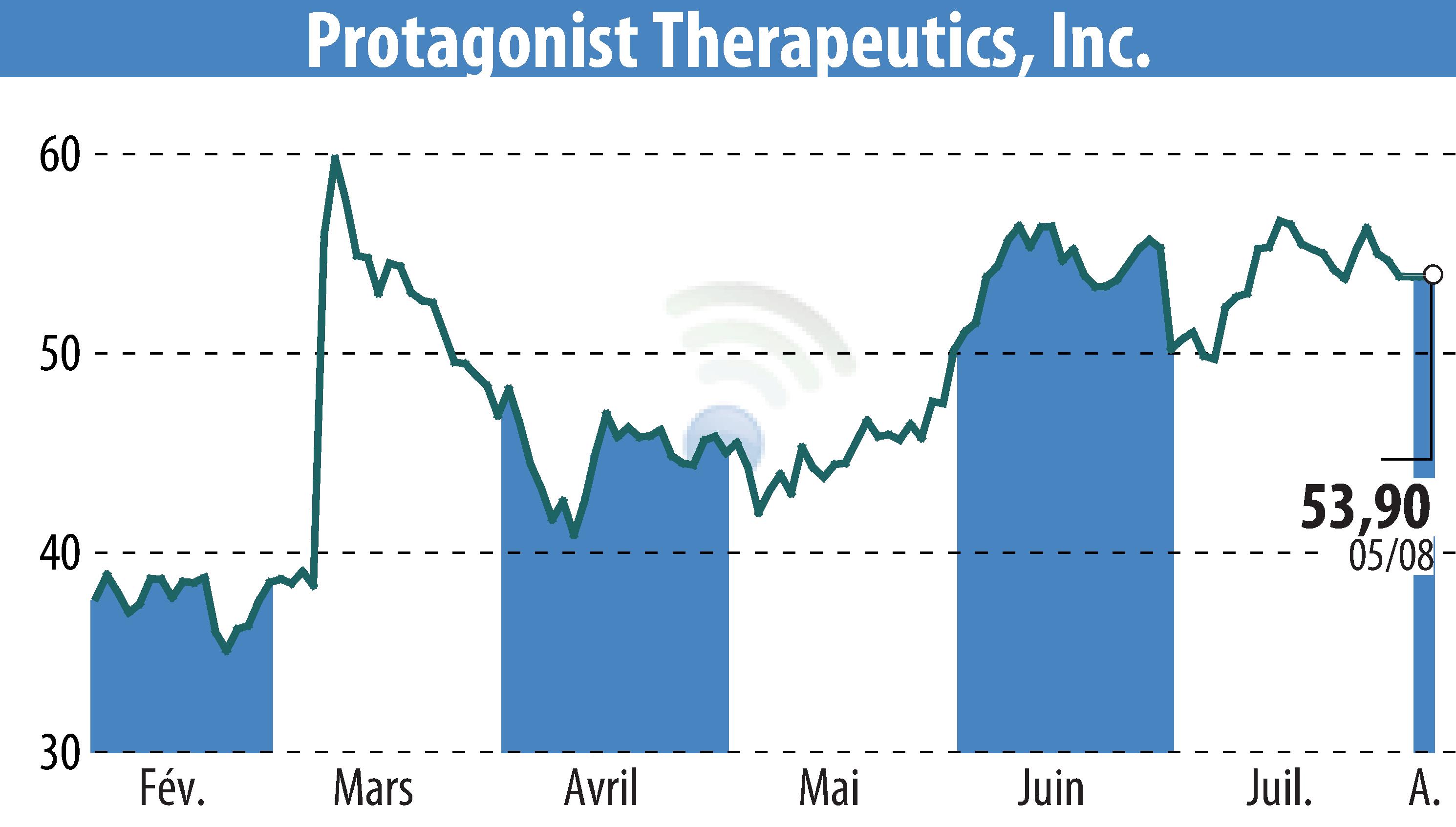
Protagonist Therapeutics has released its financial results for the second quarter of 2025, highlighting significant milestones and financial standing. A New Drug Application (NDA) for icotrokinra, targeting moderate to severe plaque psoriasis, was submitted in July to the U.S. FDA. Additionally, Phase 3 trial data of rusfertide for polycythemia vera was presented and a U.S. NDA filing is anticipated in Q4.
Financially, Protagonist reported cash and equivalents of $673 million as of June 30, expected to sustain the company until the end of 2028. However, the second quarter saw a net loss of $34.8 million, compared to $30.6 million in the same quarter of the previous year. The company continues to focus on developing its early-stage assets and advancing its clinical programs.
R. P.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Protagonist Therapeutics, Inc. news
