on Nanohale AG (ETR:FYB)
MHRA Grants Approval for Formycon and Fresenius Kabi's Otulfi®
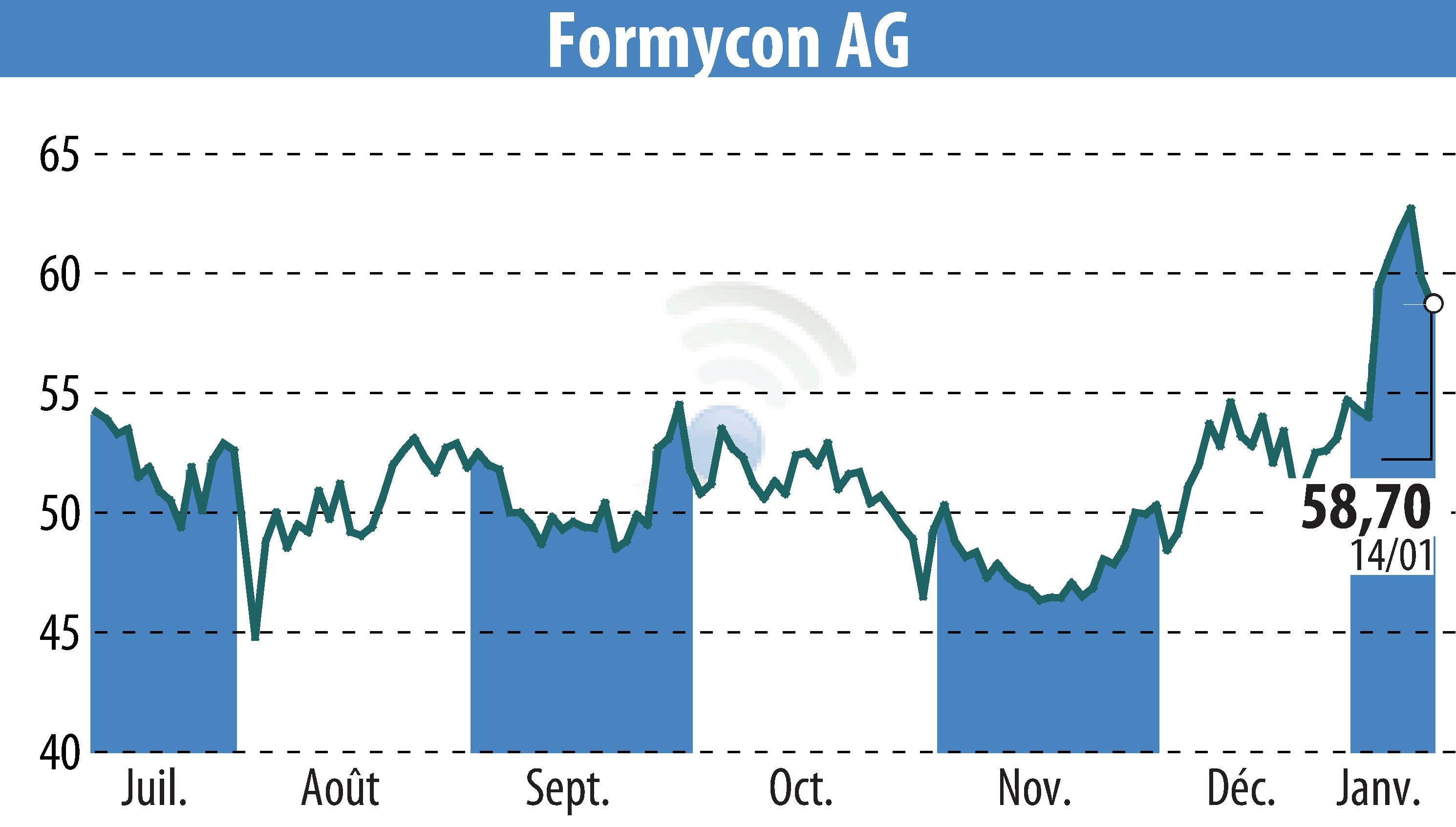
Formycon AG and Fresenius Kabi have announced the UK Medicines and Healthcare products Regulatory Agency (MHRA) approval for FYB202/Otulfi® (ustekinumab), a biosimilar to Stelara®. This approval covers both subcutaneous and intravenous forms for treating Crohn’s disease, ulcerative colitis, plaque psoriasis, and psoriatic arthritis. The authorization follows previous approvals by the FDA, European Commission, and Health Canada.
The commercialization of Otulfi® in the UK is subject to a confidential agreement with Johnson & Johnson. Ustekinumab targets cytokines interleukin-12 and interleukin-23, essential in inflammatory responses. Approval results from extensive evaluation of efficacy, safety, and manufacturing data.
Formycon, in partnership with Fresenius Kabi, aims to make high-quality, cost-efficient biosimilar treatments globally accessible, addressing the growing demand for chronic inflammatory disease treatments.
R. E.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Nanohale AG news
