on Nanohale AG (ETR:FYB)
Lotus Pharmaceutical to Commercialize Formycon's Keytruda® Biosimilar in Asia-Pacific
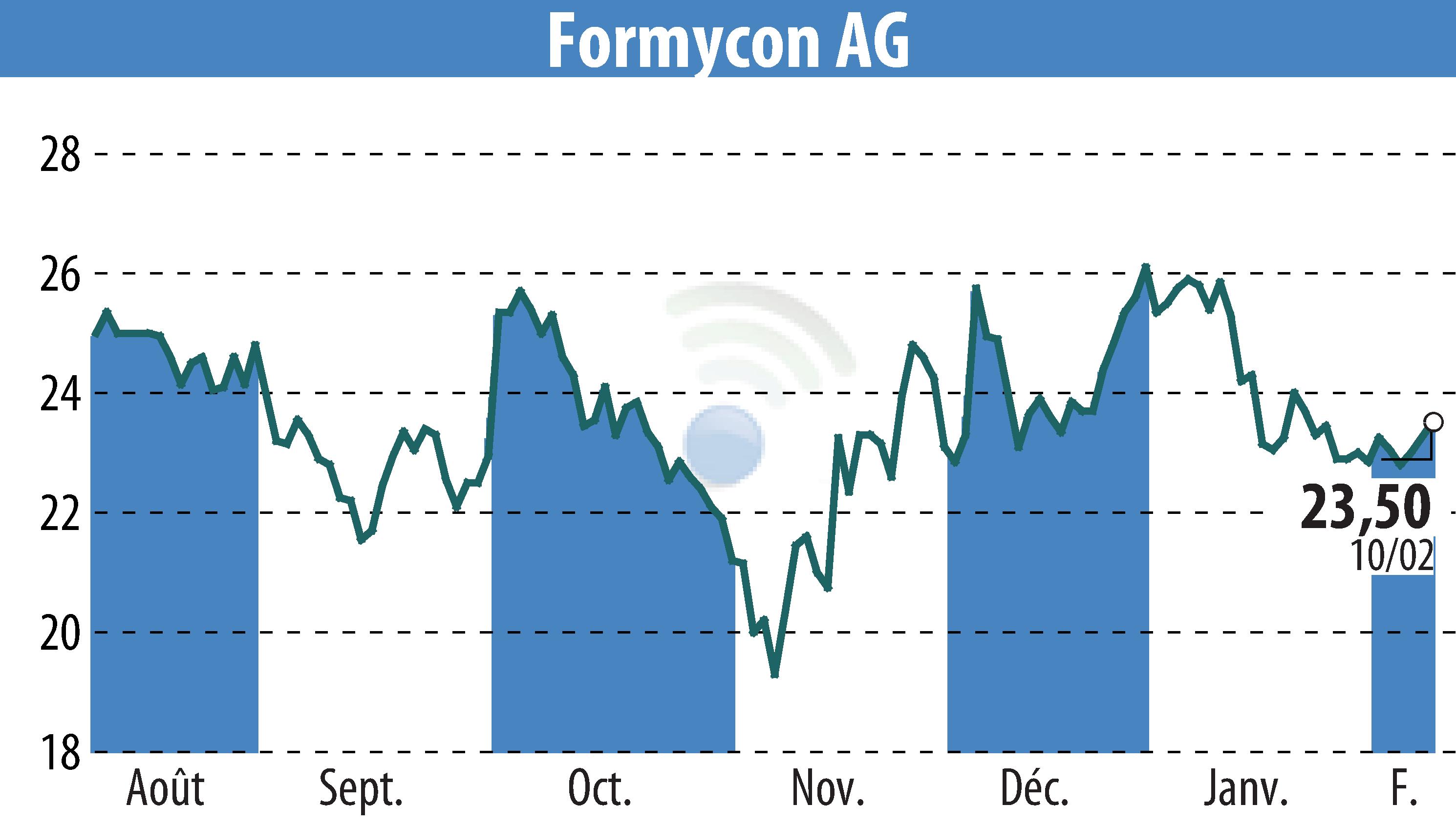
Lotus Pharmaceutical has secured an exclusive license agreement with Formycon AG to commercialize the biosimilar candidate FYB206, a version of Keytruda® (Pembrolizumab), across major parts of the Asia-Pacific region. This biosimilar aims to expand patient access to effective oncology treatments. Formycon will receive an upfront payment, additional milestone-based payments, and a share of gross profits upon the product's market launch.
FYB206 is nearing the completion of its clinical development phase, with key endpoint data expected by early 2026. Formycon and Lotus will coordinate regulatory preparations tailored to the requirements of the APAC countries. The agreement reflects both companies' commitment to providing more accessible biologic therapies and enhancing their presence in the specialty oncology market.
R. H.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Nanohale AG news
