on Nanohale AG (ETR:FYB)
Formycon Secures U.S. License for Aflibercept Biosimilar
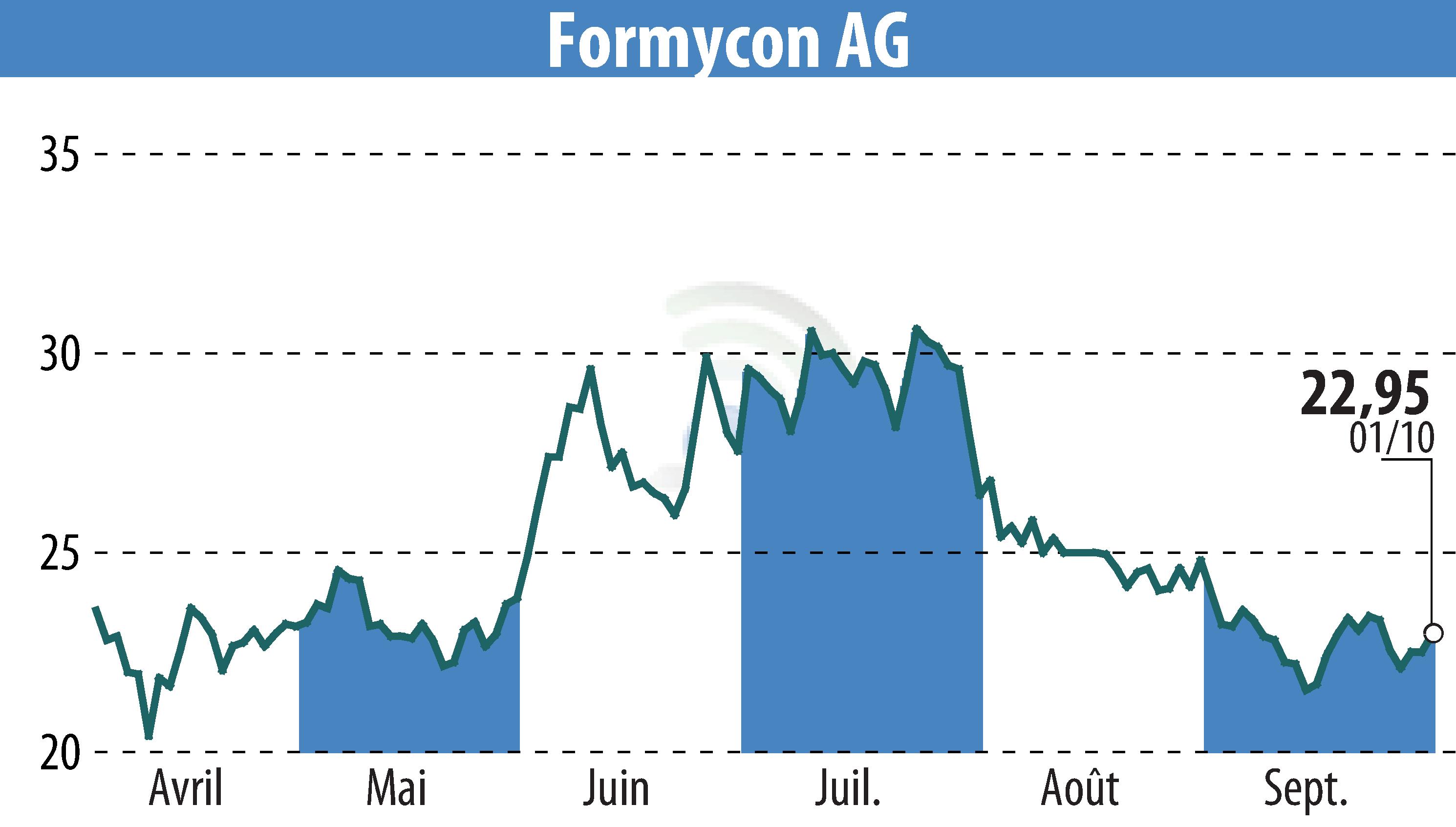
Formycon AG has announced a license agreement with Regeneron Pharmaceuticals, Inc. for its proposed biosimilar FYB203/AHZANTIVE®. This development follows the resolution of all patent disputes related to the biosimilar, aimed as an alternative to Eylea®, with litigation fully settled in the U.S. District Court for the Northern District of West Virginia.
Through a strategic partnership with Valorum Biologics, Formycon plans to launch FYB203 in the United States by Q4 2026. Having received FDA approval in July 2024, the biosimilar targets retinal diseases, leveraging aflibercept to inhibit abnormal blood vessel formation.
This agreement marks a notable achievement for Formycon, enhancing patient access to affordable, high-quality treatments in the U.S. biosimilars market.
R. E.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Nanohale AG news
