on Nanohale AG (ETR:FYB)
Formycon Completes Patient Enrollment for Keytruda® Biosimilar FYB206
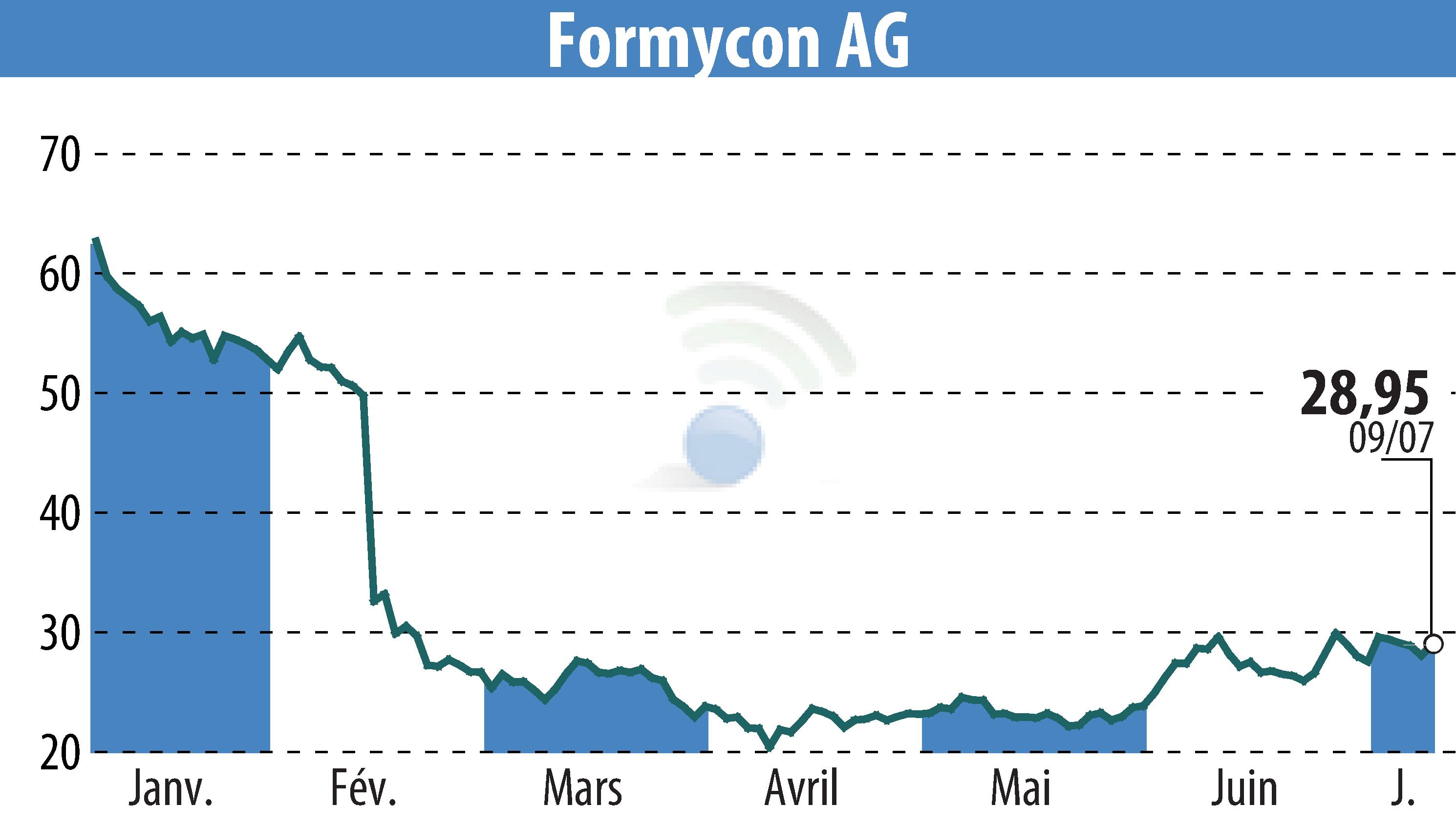
Formycon AG announced the completion of patient enrollment for its clinical pharmacokinetic study, Dahlia, concerning FYB206, a biosimilar candidate for Keytruda®. This milestone was reached with 96 participants involved. The streamlined strategy, eliminating the need for a Phase III trial, allows for accelerated development, with endpoint results anticipated in Q1 2026.
The Dahlia study, initiated in June 2024, focuses on pharmacokinetics, safety, and tolerability. Based on comprehensive data and FDA feedback, the Phase III trial was halted to expedite biosimilar availability. FYB206’s market entry is forecasted post-market exclusivity in 2029 for the USA.
R. P.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Nanohale AG news
