on Moderna, Inc. (NASDAQ:MRNA)
Moderna's RSV Vaccine, mRESVIA, Gains FDA Approval for Younger At-Risk Adults
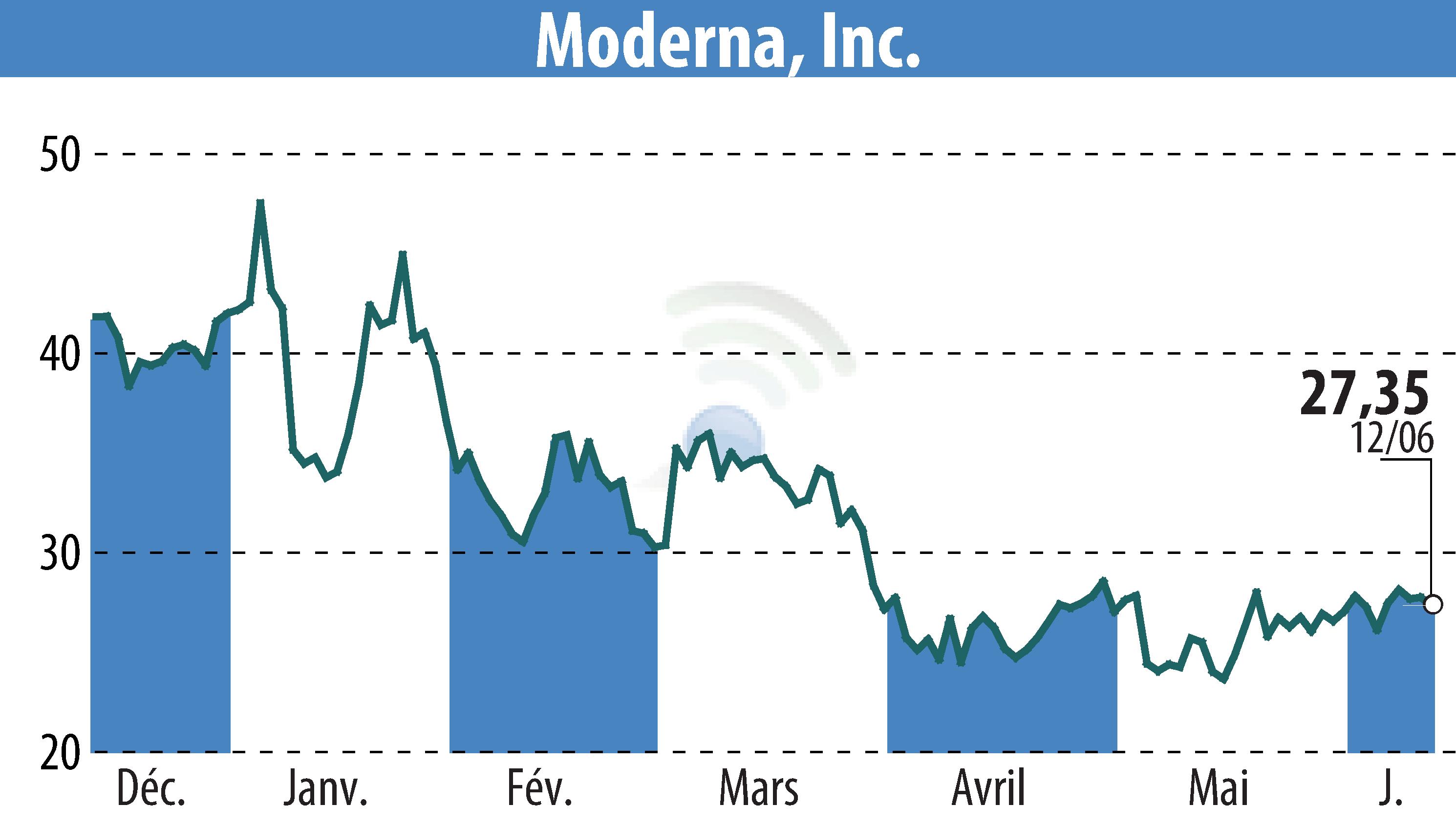
Moderna, Inc. announced FDA approval for its RSV vaccine, mRESVIA, for adults aged 18-59 who are at high risk for RSV disease. This expands the vaccine's use, which was already approved for those 60 and older. The approval addresses RSV risks in younger adults with chronic conditions, a group showing comparable disease burden to older adults.
Backed by a Phase 3 study, the vaccine demonstrated safety and strong immune responses in those with underlying conditions. Clinical trial data were shared at a CDC meeting and included in Clinical Infectious Diseases. Common side effects were injection site pain, fatigue, and headache.
Moderna plans to make mRESVIA available for the 2025-2026 respiratory virus season for all at-risk U.S. adults. The vaccine joins Moderna's portfolio of mRNA-driven solutions aimed at preventing infectious diseases.
R. H.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Moderna, Inc. news
