on Moderna, Inc. (NASDAQ:MRNA)
Moderna Seeks FDA Approval for New COVID-19 Vaccine Formula
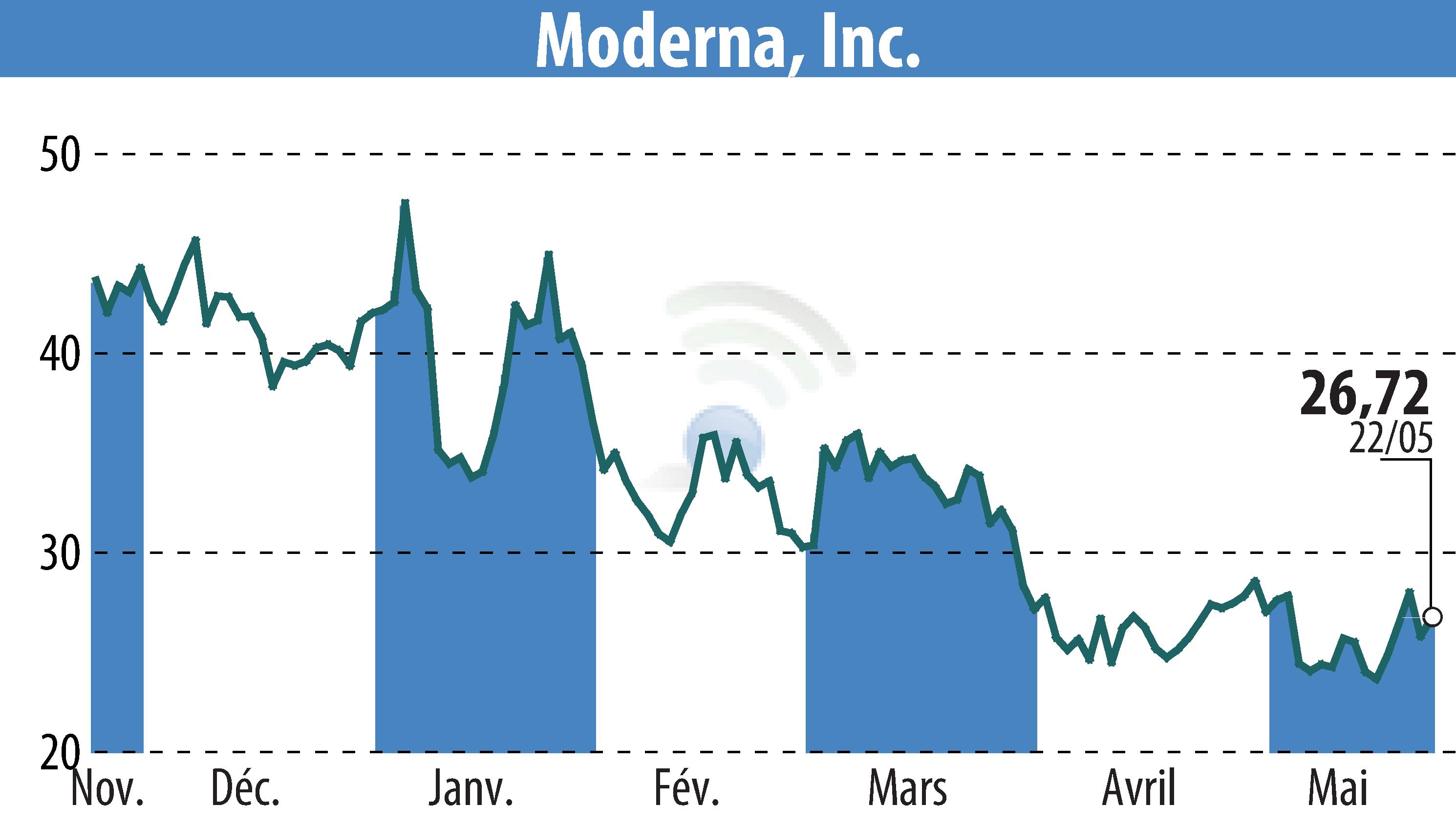
Moderna, Inc. has submitted a new application to the U.S. Food and Drug Administration (FDA) for its latest COVID-19 vaccine formula, Spikevax 2025-2026. The new vaccine targets the SARS-CoV-2 variant LP.8.1. This move aligns with FDA advice recommending COVID-19 vaccines adjustment to a monovalent JN.1 lineage, favoring the LP.8.1 variant.
The newly proposed vaccine is designed for active immunization against COVID-19 for individuals aged 12 and older. As with previous vaccines, precautions against severe allergic reactions, myocarditis, and pericarditis, among others, are noted. Potential recipients should be aware of common adverse reactions like injection site pain, headache, and fatigue.
R. P.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Moderna, Inc. news
