on MEDINCELL (EPA:MEDCL)
Medincell and Teva Submit FDA Application for New Injectable Treatment
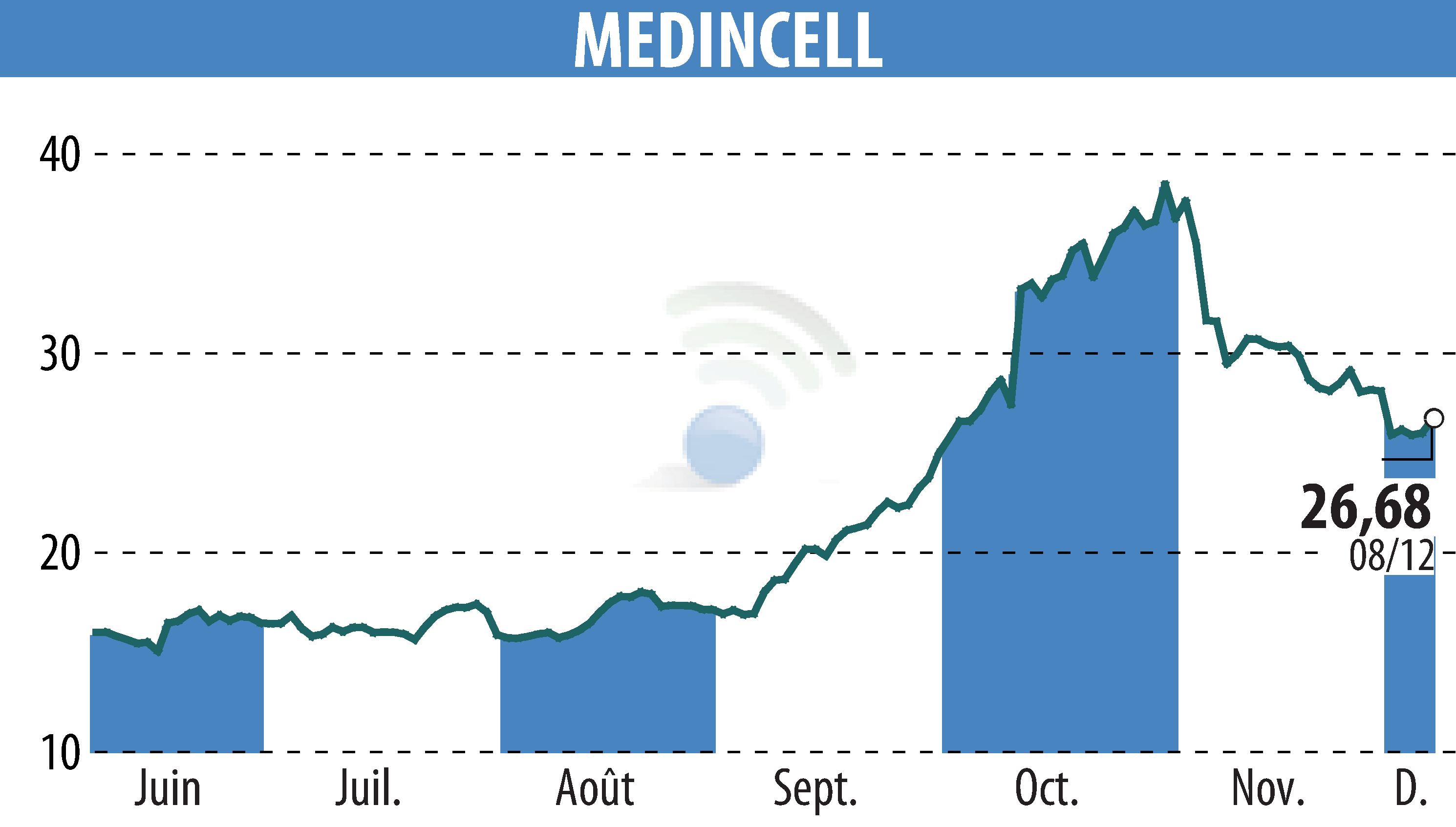
Teva Pharmaceuticals, in partnership with Medincell, has submitted a New Drug Application to the U.S. FDA for olanzapine extended-release injectable suspension (TEV-749/mdc-TJK). This medication is intended for the once-monthly treatment of schizophrenia in adults. The olanzapine long-acting injectable aims to provide efficacy comparable to regular olanzapine, while enhancing patient adherence and stability.
The FDA review typically spans two months for acceptance and a further eight months for a standard review. Olanzapine LAI is designed to bridge a critical treatment gap, addressing adherence issues common in schizophrenia management. Medincell's partnership with Teva is part of their broader strategy to develop long-acting injectables for various therapeutic areas.
R. P.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all MEDINCELL news
