on Jaguar Health, Inc. (NASDAQ:JAGX)
Jaguar Health Seeks EMA Approval for Canalevia to Treat Dog Diarrhea
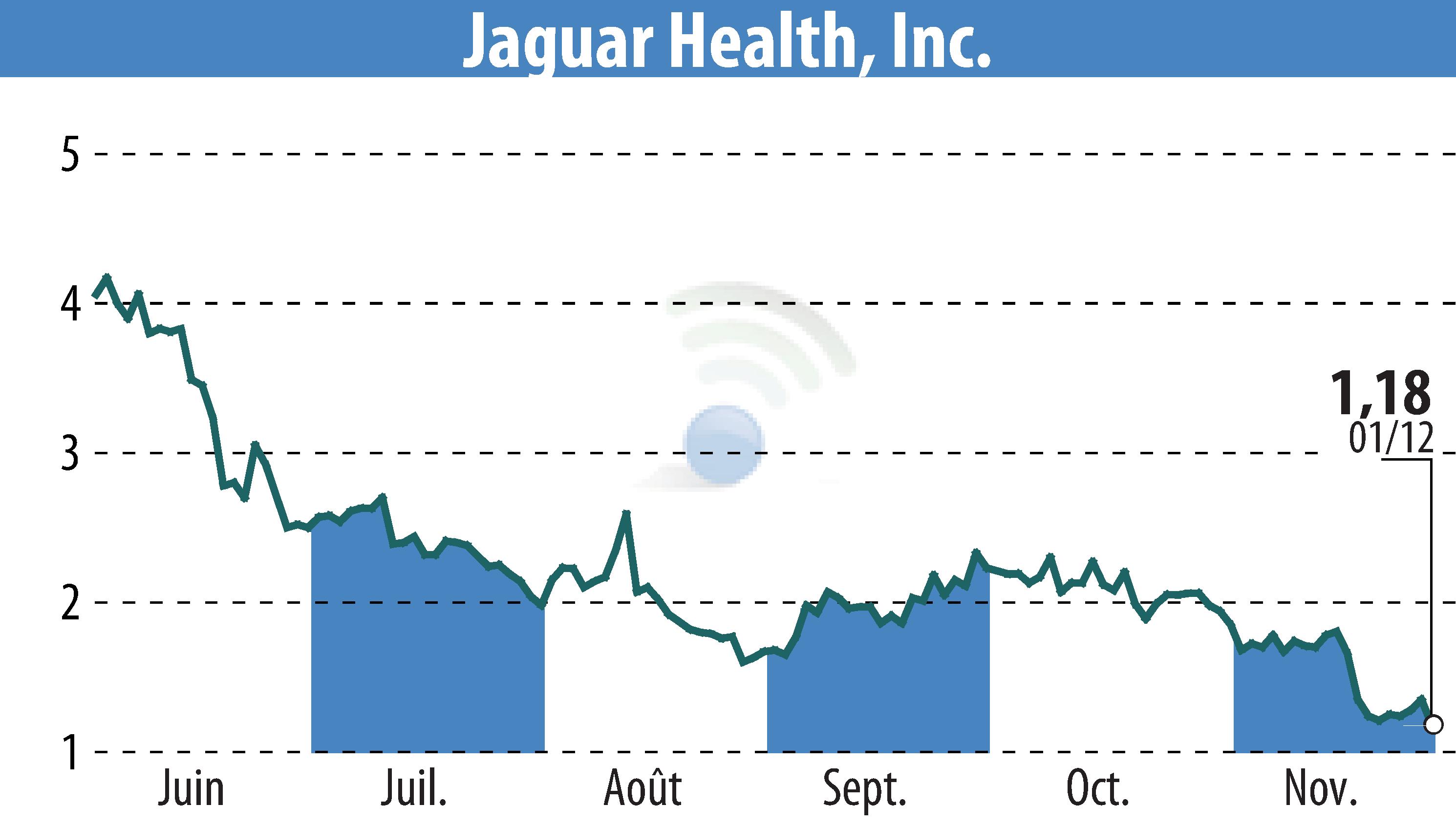
Jaguar Health, Inc. has taken a critical step towards expanding its canine gastrointestinal treatment, Canalevia, in the European Union. The company has submitted a request to the European Medicines Agency (EMA) for scientific advice concerning the approval pathway for Canalevia, aimed at treating general diarrhea in dogs. This submission comes on the heels of a study involving 200 dogs, which initially did not meet its primary endpoint. However, a revised analysis indicated better outcomes for dogs treated with Canalevia compared to placebo.
Canalevia, conditionally approved in the U.S. for chemotherapy-induced diarrhea, represents a novel non-antibiotic approach to treating one of the most common veterinary issues. Jaguar aims to secure a partner for global development and commercialization, especially given the lack of FDA-approved options for general canine diarrhea.
R. H.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Jaguar Health, Inc. news
