on Jaguar Health, Inc. (NASDAQ:JAGX)
Jaguar Health Advances Crofelemer Development for Intestinal Disorders
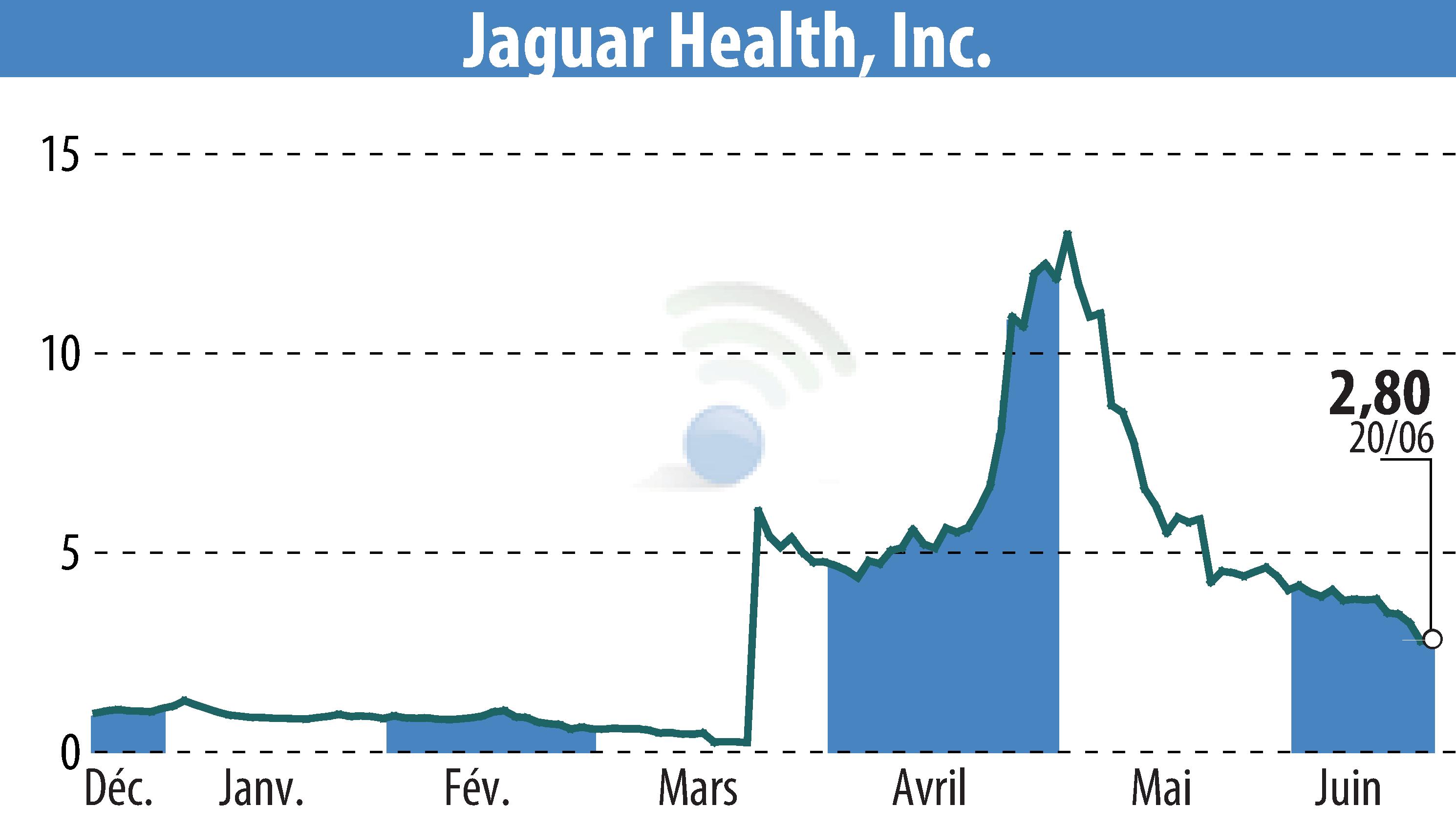
Jaguar Health has made progress in its orphan disease program concerning intestinal failure. The company is targeting pediatric patients with microvillus inclusion disease (MVID), aiming to reduce reliance on parenteral nutrition through the plant-derived drug, crofelemer. Enrollment in the Phase 2 study has reached 25%, with promising proof-of-concept results showing a reduction in required parenteral nutrition.
Data from initial trials indicate crofelemer reduced total parenteral nutrition needs by 12.5% to 27%. Jaguar Health continues its strategy to seek business partnerships for non-dilutive funding and commercialization of its products. The company anticipates expedited regulatory approval processes, leveraging these promising early results.
CEO Lisa Conte expressed optimism based on recent meetings and ongoing trials in multiple regions, including the U.S., EU, and the Middle East. Completion of the Phase 2 study in pediatric MVID patients is expected by mid-2026, as the company continues to pursue regulatory pathways.
R. H.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Jaguar Health, Inc. news
