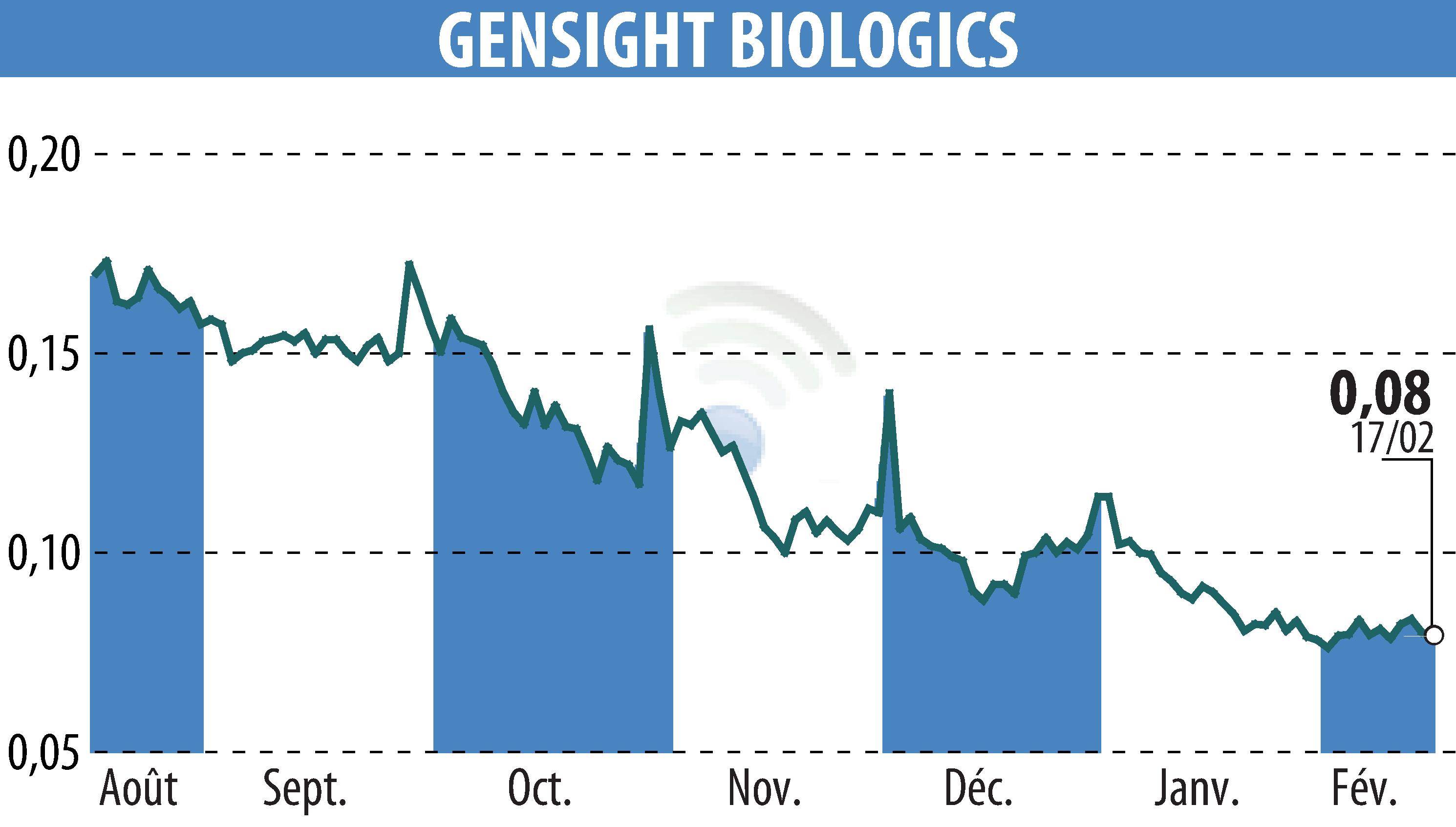
on GENSIGHT BIOLOGICS S.A. (EPA:SIGHT)
GenSight Biologics Strengthens Regulatory Leadership with Key Appointments
GenSight Biologics has made strategic additions to its Regulatory Affairs & Quality team with the appointments of Fang Li, Ph.D., RAC, as Chief Regulatory Affairs & Quality Officer, and Sabrina Chekroun, Pharm.D., as Senior Vice President. These appointments aim to bolster the company's regulatory strategy in the US and Europe following recent regulatory achievements.
Fang Li, based in the U.S., brings over 30 years of drug development experience, including senior roles in regulatory affairs across notable biotech firms. Sabrina Chekroun, located in France, offers extensive knowledge in global regulatory strategies with a strong focus on orphan drugs and rare diseases.
This expansion comes as GenSight prepares to launch a new global Phase III trial, enhancing its capacity to deliver innovative gene therapies for retinal neurodegenerative diseases.
R. P.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all GENSIGHT BIOLOGICS S.A. news
