on GENSIGHT BIOLOGICS S.A. (EPA:SIGHT)
GenSight Biologics Begins REVISE Study for GS010 Therapy
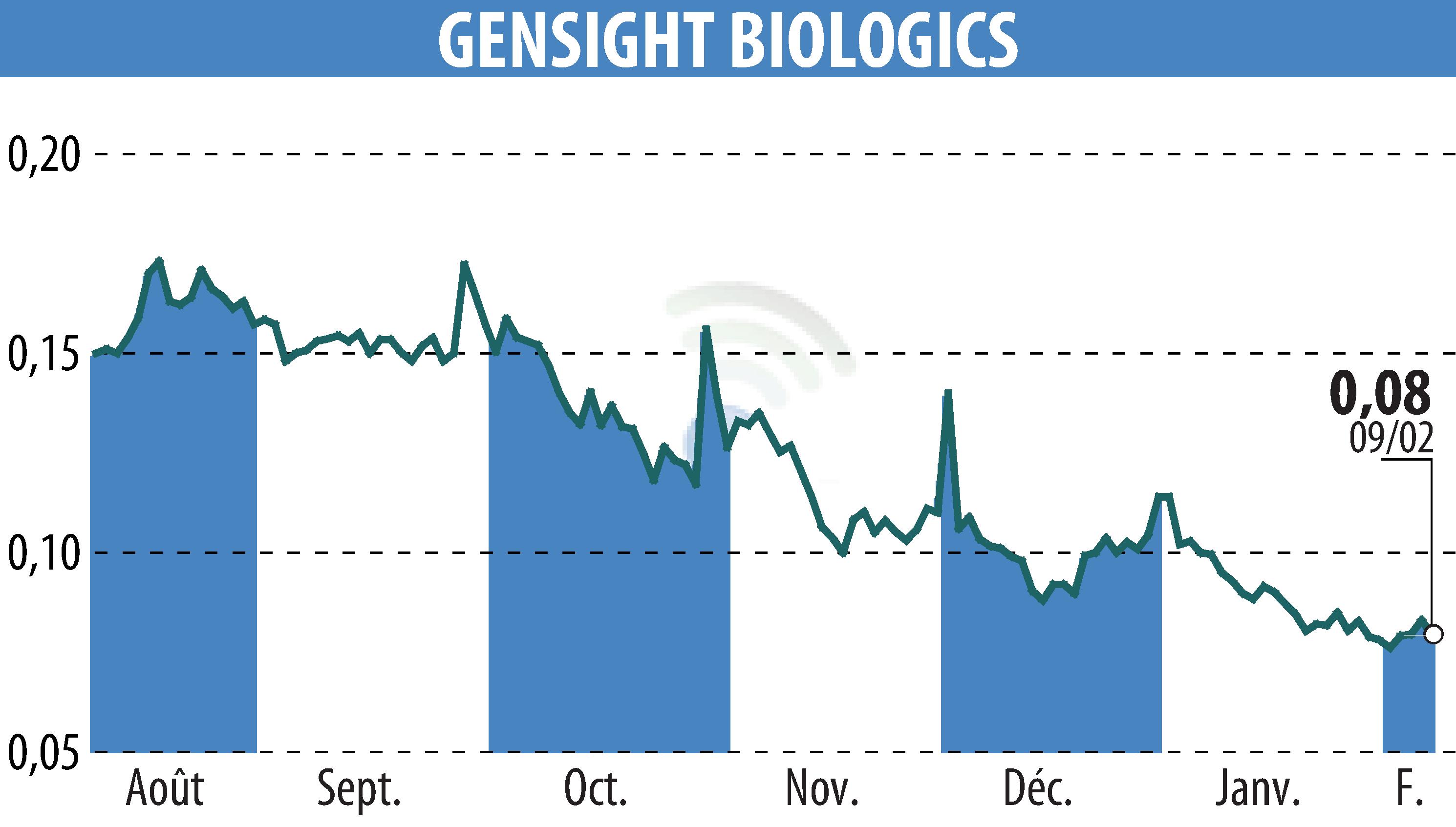
The 15-20 National Hospital in Paris and biopharma company GenSight Biologics have initiated the treatment of the first patient in the GS010/LUMEVOQ® REVISE study. Approved by France's ANSM, this dose-ranging study will include 14 patients. The study advances ongoing efforts to develop transformative treatments for rare diseases like Leber Hereditary Optic Neuropathy (LHON).
Currently, the 15-20 National Hospital is the sole European facility conducting clinical studies with GS010, a gene therapy candidate aimed at treating LHON caused by ND4 mitochondrial mutations. The study's initiation highlights the hospital's support for significant scientific advancements. The French ANSM authorized both the REVISE study and an early access program in December 2025.
GS010 has not yet received marketing approval. However, its clinical development progresses, underscoring the urgency to address unmet medical needs in LHON patients.
R. H.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all GENSIGHT BIOLOGICS S.A. news
