on GENSIGHT BIOLOGICS S.A. (EPA:SIGHT)
First Steps of the REVISED Clinical Study on GS010/LUMEVOQ®
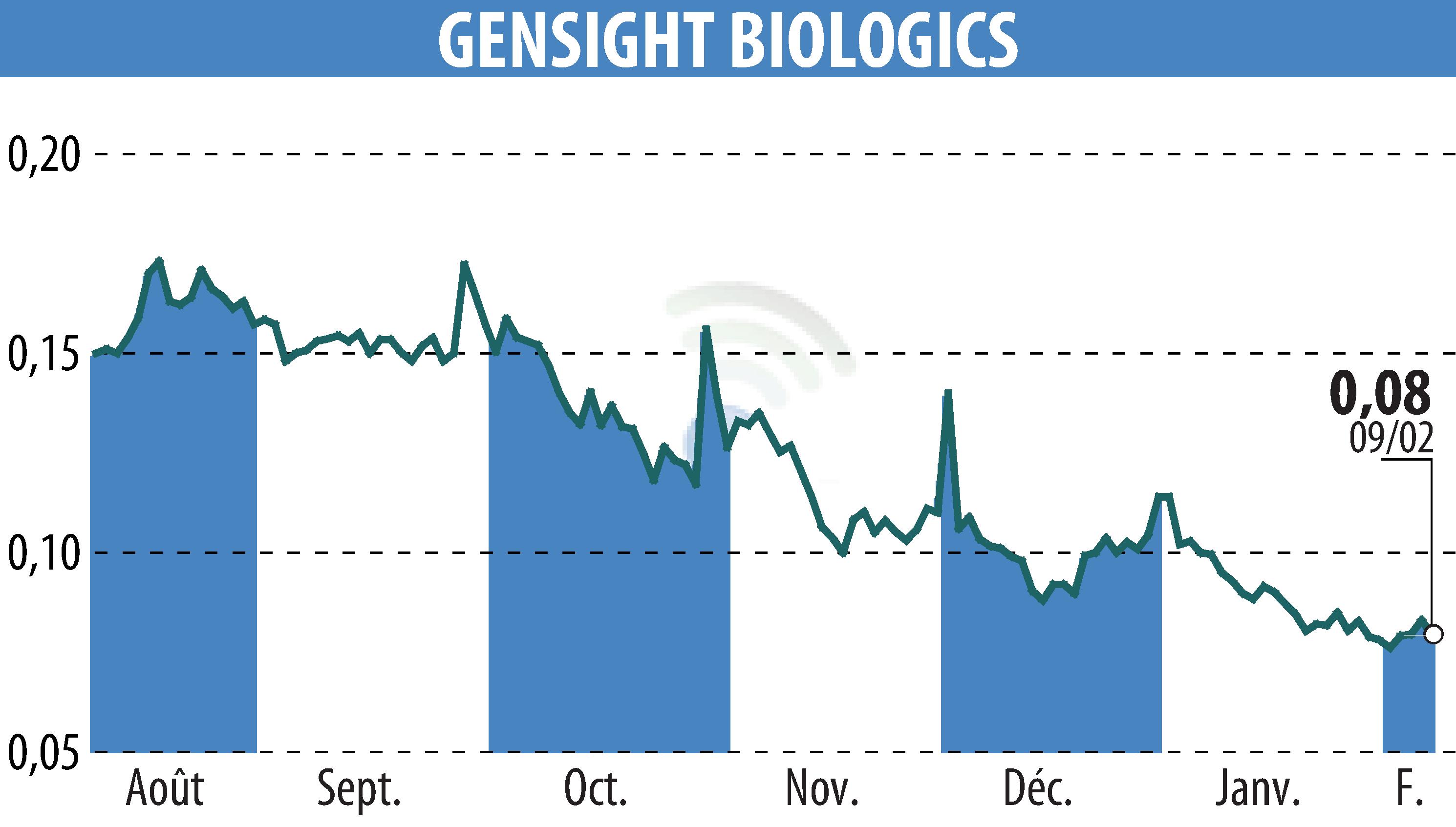
On February 10, 2026, the biopharmaceutical company GenSight Biologics and the Quinze-Vingts National Hospital in Paris announced the treatment of the first patient in the REVISE study. This research, approved in December 2025, is examining the potential of GS010/LUMEVOQ® for Leber hereditary optic neuropathy (LHON), caused by a mutation in the ND4 gene.
The study, conducted exclusively in a hospital setting, will include 14 French patients. The site is also the only one in Europe to administer GS010 under Compassionate Access Authorization. The objective is to evaluate two doses of the gene therapy. Eligible French patients will be given priority for inclusion in the study.
GenSight Biologics plans to launch a new Phase III trial by the end of 2026, highlighting the importance of treating this rare disease. The partnership strengthens their shared mission to advance treatments for rare diseases.
R. P.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all GENSIGHT BIOLOGICS S.A. news
