on GENFIT (EPA:GNFT)
GENFIT's Positive Phase 2 Results for Elafibranor in PSC Announced
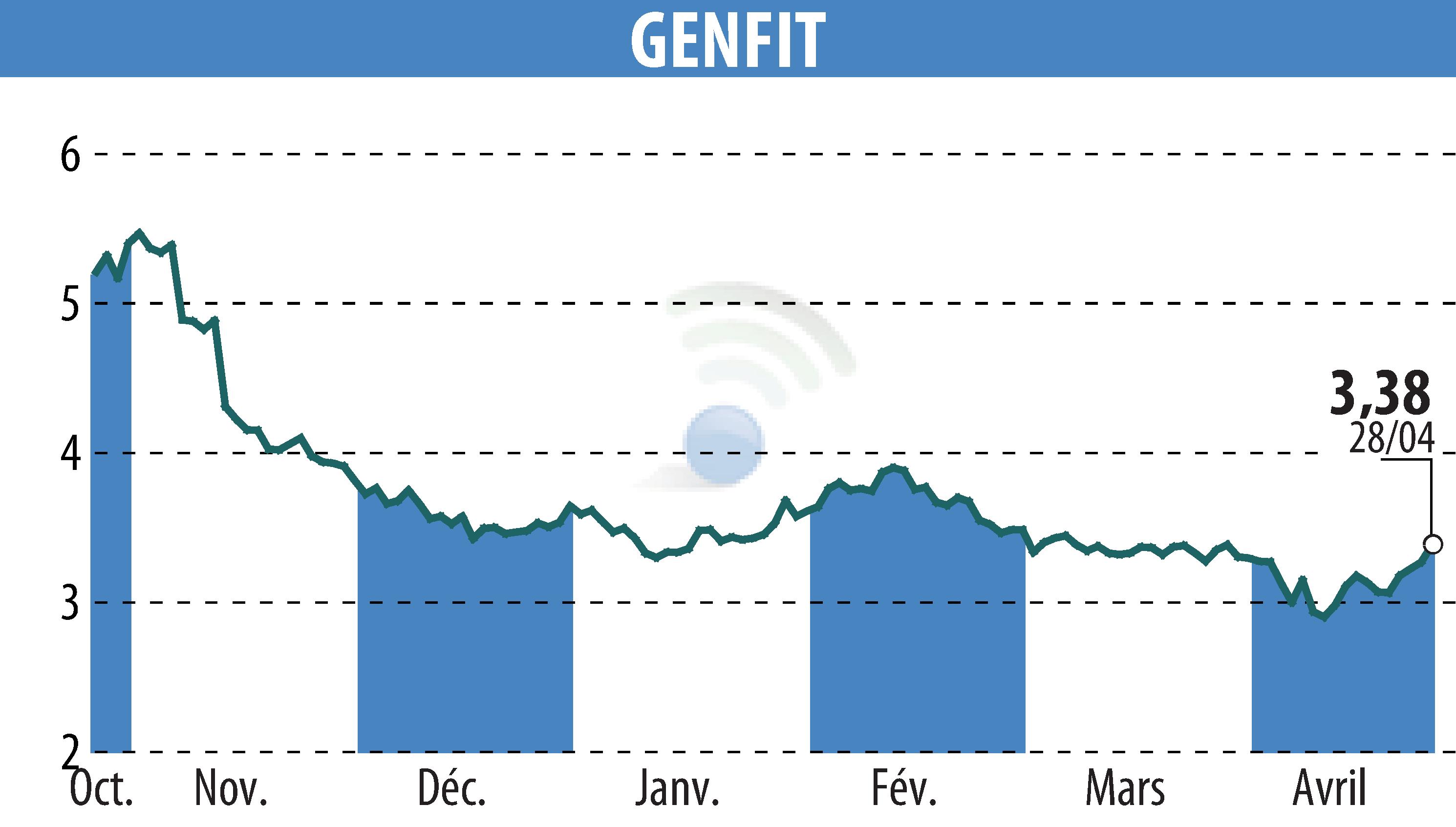
On April 28, 2025, GENFIT revealed that Ipsen would present positive Phase 2 data on elafibranor for Primary Sclerosing Cholangitis (PSC) at the EASL Congress. Elafibranor showed significant efficacy, notably reducing alkaline phosphatase levels in the ELMWOOD trial. Patients taking 80 mg and 120 mg dosages experienced notable improvements as early as week 4 and stabilization of Enhanced Liver Fibrosis at week 12.
Elafibranor, known as Iqirvo®, is already marketed for Primary Biliary Cholangitis (PBC) and is noted for its favorable safety profile. The drug was developed by GENFIT and licensed to Ipsen. GENFIT continues to focus on liver diseases, believing in elafibranor's potential to address significant unmet needs.
R. E.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all GENFIT news
