on Nanohale AG (ETR:FYB)
Formycon and Fresenius Kabi Achieve FDA Interchangeability Approval for FYB202/Otulfi®
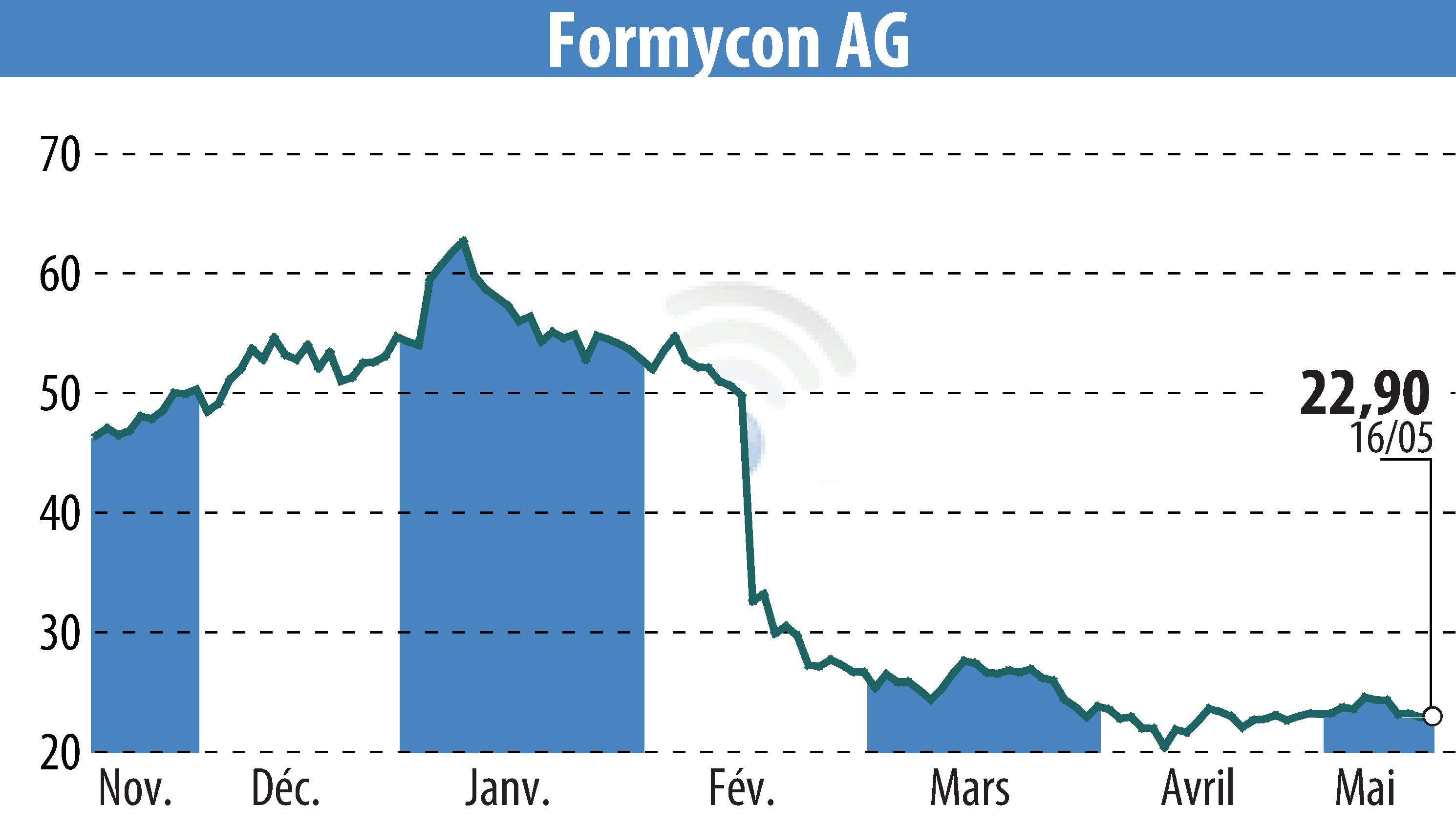
Formycon AG and Fresenius Kabi announced that the U.S. FDA has granted interchangeability for FYB202/Otulfi® (ustekinumab-aauz) with Stelara®. Effective April 2025, Otulfi® can be substituted without prescriber approval, facilitating patient access. Otulfi® was launched in the U.S. in March 2025 in various dosages.
The CMS has assigned a specific billing code for Otulfi®, streamlining insurance claims and reimbursements. Dr. Stefan Glombitza, CEO of Formycon, highlighted the interchangeability status as a testament to the product's quality and a driver for market uptake.
FDA's approval was based on comprehensive data demonstrating Otulfi®'s comparable efficacy and safety to Stelara®. These moves align with Formycon's mission to provide affordable treatment alternatives.
R. H.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Nanohale AG news
