on DBV TECHNOLOGIES (EPA:DBV)
DBV Technologies: Encouraging Results from the VITESSE Study on the Peanut Allergy Patch
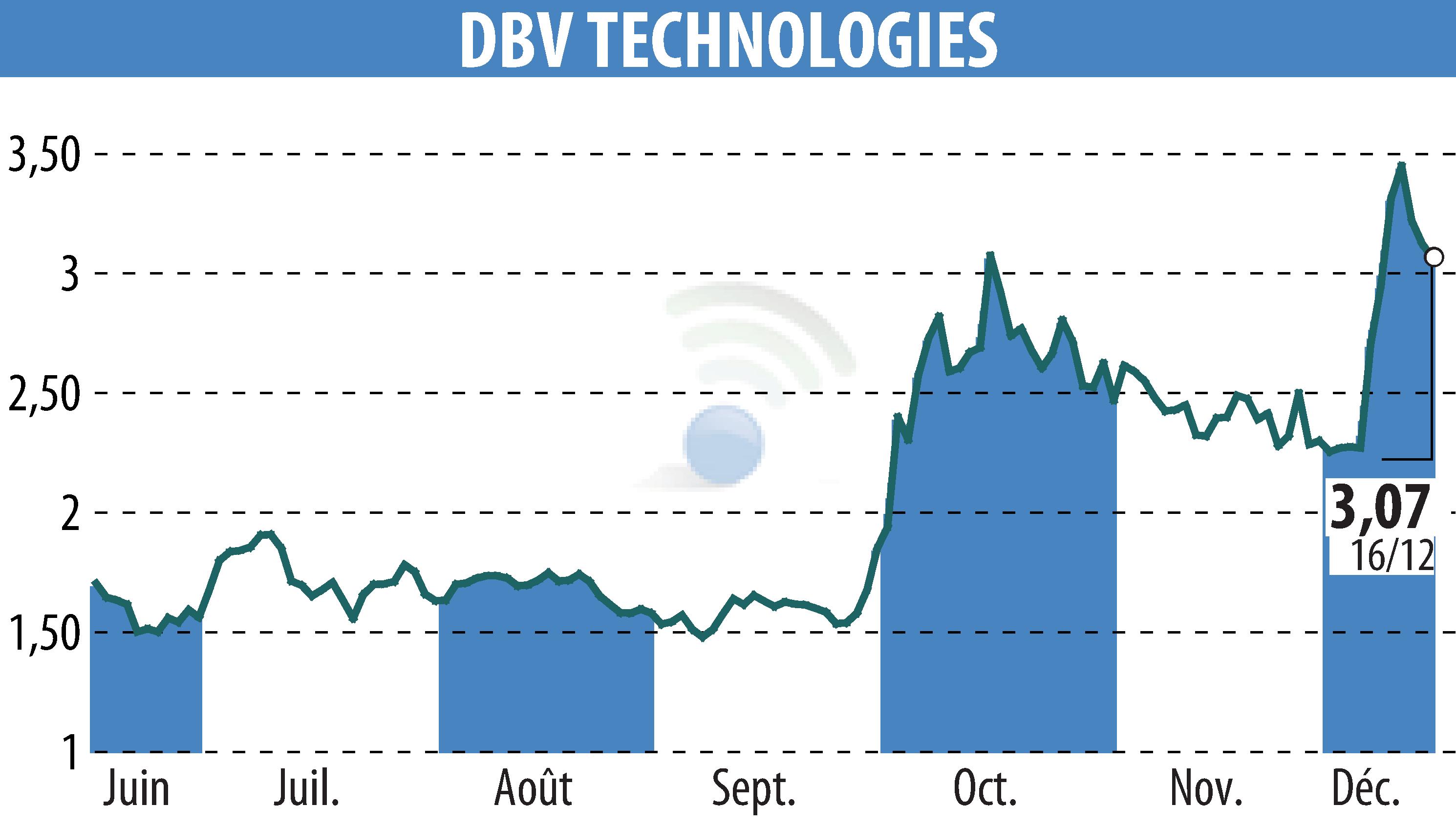
DBV Technologies has published positive preliminary results from its Phase 3 VITESSE study of the VIASKIN® Peanut patch. Conducted in children aged 4 to 7 years with peanut allergy, the study met its primary endpoint, demonstrating a 24.5% difference in response between the placebo and active groups, exceeding the expected threshold of 15%.
Among the children treated, 46.6% responded positively to the patch after 12 months, compared to 14.8% in the placebo group. The safety of the treatment was confirmed, with mainly mild skin reactions.
These results support a planned Biologics License Application in the first half of 2026. A conference call will provide further details on these advances. The VIASKIN patch could become a non-invasive and well-tolerated solution for young patients.
R. P.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all DBV TECHNOLOGIES news
