on BIOSYNEX (EPA:ALBIO)
BIOSYNEX announces dual FDA filing for an innovative syphilis test
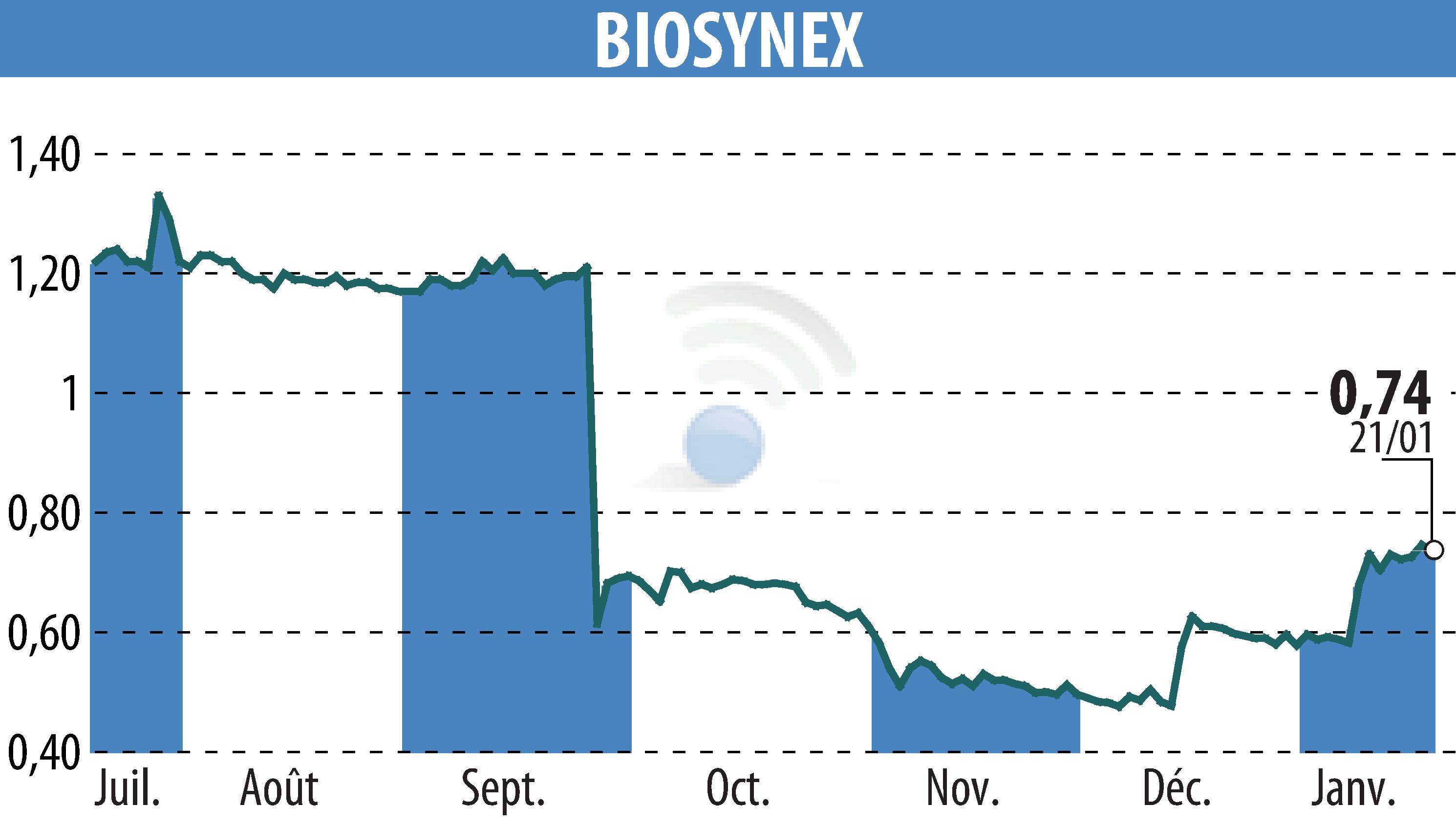
On January 22, 2026, Chembio Diagnostic Systems Inc., a subsidiary of BIOSYNEX, announced the FDA submission of its DPP® Syphilis TnT test. Recognized as an innovative device by the FDA, this test aims to distinguish between active and recurrent syphilis, facilitating immediate treatment. This tool utilizes Dual-Path Platform technology for results in 15 minutes and is suitable for decentralized settings, such as public health centers and community programs.
The FDA's Breakthrough Device designation, granted in July 2025, underscores the urgent need for rapid diagnostics in the United States, as syphilis continues to rise, particularly among pregnant women and underserved communities. If approved, this test would be the first to be used outside of accredited laboratories, directly accessible to patients, thus enabling rapid treatment decisions.
R. P.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all BIOSYNEX news
