on Bayer Aktiengesellschaft (ETR:BAYN)
Bayer's Asundexian Achieves Key Endpoints in OCEANIC-STROKE Study
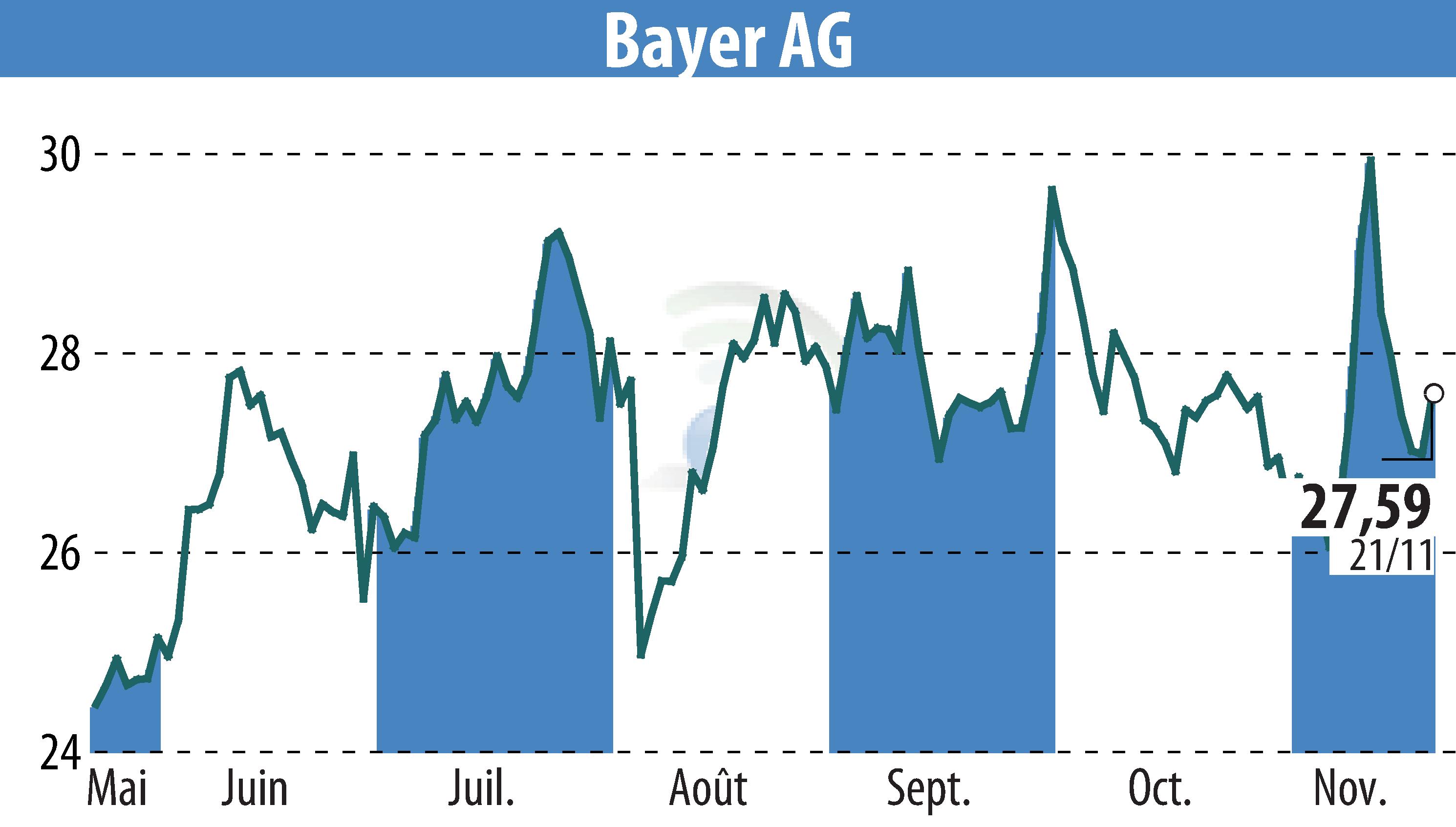
Bayer Aktiengesellschaft has announced successful results from the Phase III OCEANIC-STROKE study. The investigational oral FXIa inhibitor, asundexian, met primary efficacy and safety goals. It significantly reduced ischemic stroke risk compared to placebo when combined with antiplatelet therapy in patients post non-cardioembolic ischemic stroke or high-risk transient ischemic attack (TIA). Importantly, there was no increase in major bleeding risk associated with asundexian.
Over 12,300 patients participated in this multicenter, double-blind study. Bayer plans to engage with global health authorities to seek marketing authorization. Detailed findings are slated for presentation at an upcoming scientific congress. Though promising, asundexian remains unapproved by health authorities for any country or indication.
R. H.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Bayer Aktiengesellschaft news
