on ABIVAX (EPA:ABVX)
Abivax Presents Promising 8-Week ABTECT Trial Results
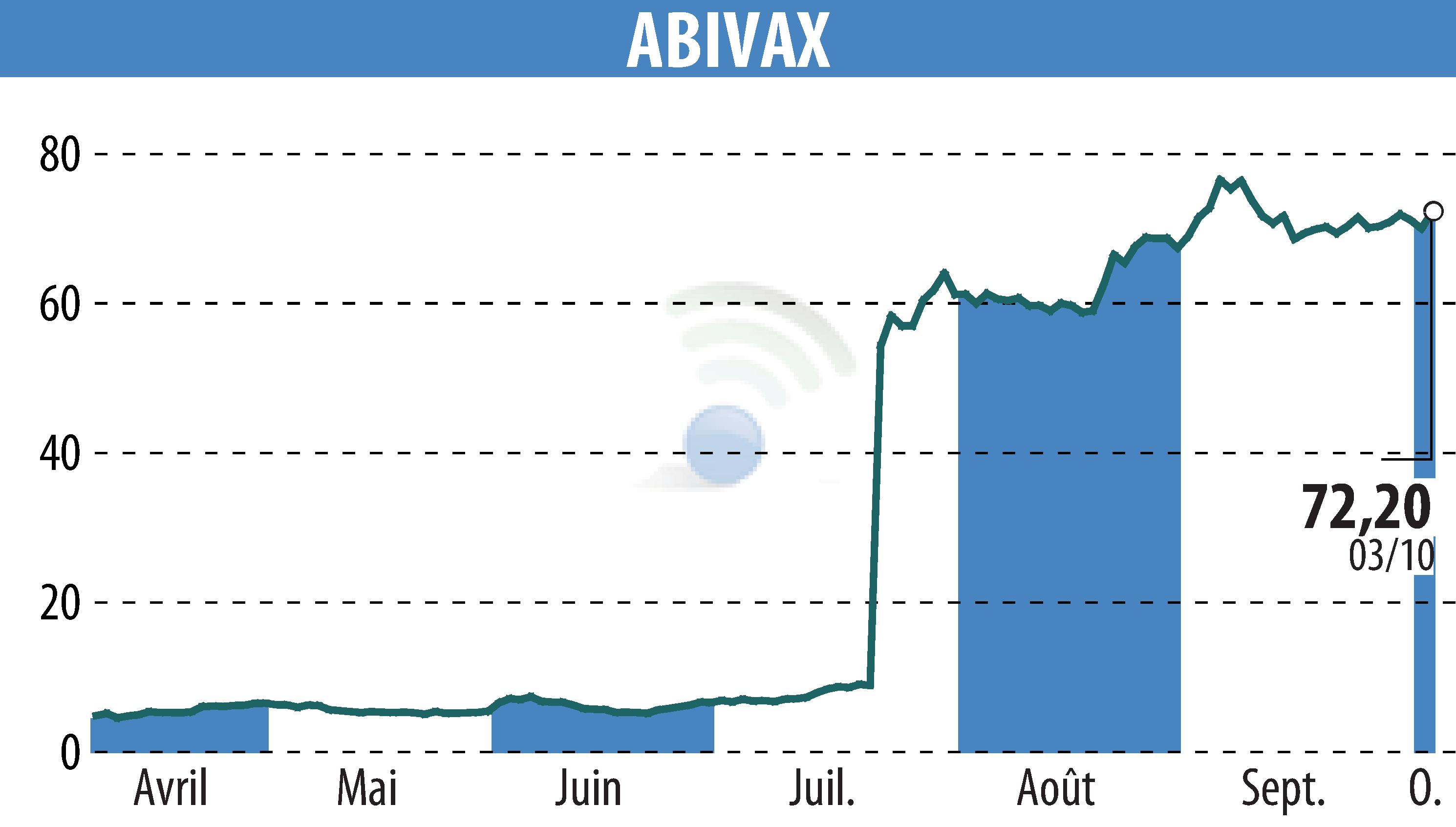
Abivax SA has unveiled promising results from their 8-week ABTECT Phase 3 trials. The trials focused on using a 50 mg once-daily dose of obefazimod for treating moderate-to-severe ulcerative colitis. This dosage resulted in a 16.4% placebo-adjusted clinical remission rate by Week 8, meeting both primary and secondary endpoints in ABTECT 1 and 2.
The study enrolled a challenging patient population. Notably, 47% had inadequate responses to previous advanced therapies, including 21% who had failed JAK inhibitor therapy. Obefazimod was well-tolerated across both 25mg and 50mg doses, with no new safety concerns.
No serious infections or malignancies emerged, despite common adverse effects like headaches and nausea. The results emphasize obefazimod's potential for patients with refractory diseases. A follow-up presentation is scheduled for October 6, with an investor call to discuss these findings further.
R. E.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all ABIVAX news
